AG - Biological basis for biohybrid implants
The musculoskeletal system as a clinical and scientific field of activity
Diseases of the musculoskeletal system are of the highest socio-economic relevance. Today, around 80% of the population suffer from neck, shoulder or back pain in the course of their lives. This finding will become even more relevant in the future due to increasing life expectancy. Innovative treatment approaches are necessary to enable optimal therapeutic success in the future with the shortest possible convalescence phase and the result of long-term full functional recovery. Regenerative medicine promises such innovative solutions. The successes in stem cell research in particular (Nobel Prize 2012) provide additional momentum for this. Among other things, the body's own stem cells are available for research and possibly for clinical use. Further successes will result from the further development of biomedical implants. Here we are concentrating in particular on the further development of musculoskeletal implants. The group's work is located at the interface between basic science and clinical translation, as we translate results from our research - e.g. on signaling pathways - into application-oriented solutions - e.g. within a DFG-funded research group, see below.
Contributions to the MHH research focus "Transplantation, Regeneration"
Mesenchymal stromal/stem cells (MSCs) are the body's own stem cells in the skeletal system. Alongside haematopoietic stem cells, they form the second most important group of tissue-specific stem cells and, like these, are found in the bone marrow, but also in various other tissues of the body and in birth-associated tissues. In vitro and in vivo, they can be differentiated into cells of the mesenchymal lineage: bone, cartilage, tendon and ligament, adipose tissue or muscle cells. In addition, MSCs are immunoprivileged and have immunomodulatory (especially immunosuppressive) functions. These properties are essentially based on the effects of soluble factors released by MSCs. MSCs are therefore highly medically relevant: After hematopoietic stem cells, they are most frequently used in clinical trials and for very different diseases, including rejection reactions ("graft versus host disease") and autoimmune diseases, while applications in the skeletal system account for less than a quarter of all clinical trials.
Thus, MSCs represent a highly attractive cell source, both in terms of basic science and in their application in biomedical research and engineering. The work of my group is intended to help identify the importance of these cells for the areas of application mentioned and to make the results obtained in research and Clinical Department more meaningful, reproducible and predictable in the future. To this end, we investigate the function of human mesenchymal stem cells under normal and inflammatory conditions and develop novel isolation and cultivation strategies. The focus is on understanding stem cell functions and networks of signaling molecules. Last year, the RENEW-MSC working group at the MHH was able to publish the scientific results of a joint research project in a renowned journal, "Cytotherapy", and offers a lecture series for students.
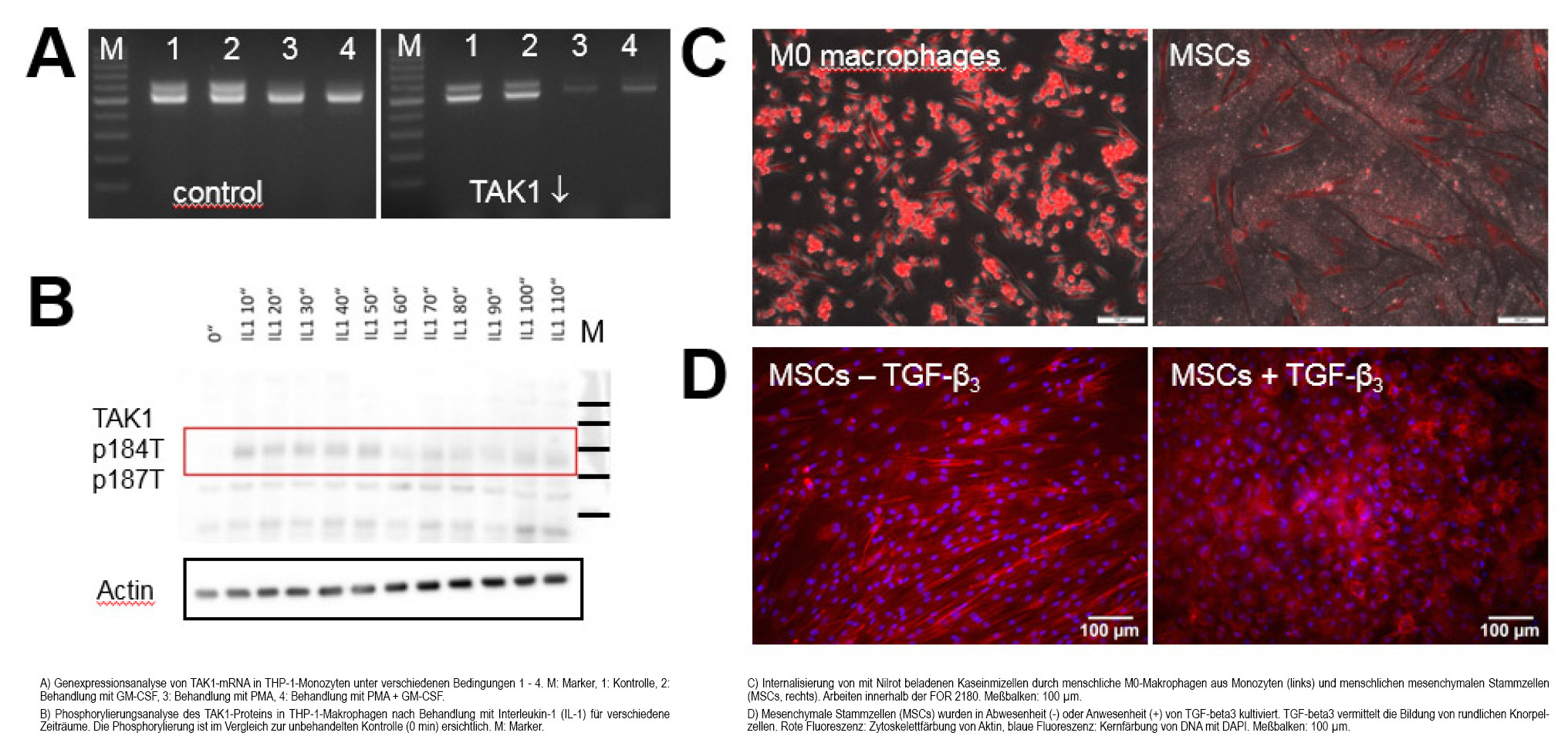
Signaling pathways in regeneration: A molecular mechanism for tendon cell formation. Genetically modified MSCs differentiated in vitro into tendon-like cells. Implantation in vivo enabled cell-mediated gene therapy of a segmental Achilles tendon defect in rats. After intramuscular implantation, ectopic tendon formation took place or even tendon-bone junctions (entheses) were formed. This work is the central basis for today's funding in the DFG Research Unit 2180 "Graded implants for tendon-bone connections", see chapter "Graded implants".
Signaling pathways in inflammation: TAK1 is involved in the development of rheumatoid arthritis. The MAP3 kinase TAK1 (Transforming Growth Factor-b Activated Kinase 1) is a member of the mitogen-activated protein kinase family. It has integrative functions in the transmission of TGF-b growth factor signals as well as in infections and inflammatory reactions. It has been shown that TAK1 is involved in the development of fibrosis and influences the differentiation of tissue-resident stem cells and immune cells. Rheumatoid arthritis is a chronic inflammatory disease that leads to progressive joint destruction. Although new biotherapies have revolutionized treatment, they often have side effects that necessitate further development of alternative anti-inflammatory strategies. In a mouse model (collagen-induced arthritis in DBA/1 mice), we were able to show that systemic inhibition of TAK1 led to remission of disease signs. This finding could enable the development of anti-inflammatory strategies as an alternative to current biotherapies.
Contributions to the MHH research focus "Biomedical Engineering, Implants"
Graded implants. Biomedical implant research has so far concentrated on implants for homogeneously structured tissue types, e.g. bone. Less well researched are implants for areas that are located between tissues with very different properties. Natural tissue transitions exhibit multiple gradients: Gradients of structure, composition and consequent functionality. Such tissue transitions play a special role in the field of orthopaedics. Their functionality is often impaired by pathological processes. Ongoing treatment costs, revision operations and yet unsatisfactory clinical results represent a considerable problem. The DFG-funded research group FOR 2180 "Graded implants for tendon-bone connections" will address the shoulder as an example: www.gradierte-implantate.de. The aim is to demonstrate the feasibility in principle and model production of a new type of biodegradable and multi-graded implant for future use at the tendon-bone junction of the rotator cuff. FOR 2180 spans specialist areas from engineering, natural and life sciences and medicine. Three universities in Lower Saxony - MHH, Leibniz University and Braunschweig University of Technology - are involved. The head of the working group is the spokesperson for this joint project.
Interaction of biomaterials and the immune system. Previous results of FOR 2180 show a massive foreign body reaction and fibrosis. We assume that the effects of inflammation and immune reactions should be taken more into account in future developments of regenerative therapies including biomaterials and implants. For example, anti-inflammatory and immunomodulatory approaches could in many cases sustainably promote or enable successful regeneration, including the potentiation of stem cell and growth factor effects. In the future, we would like to develop tailor-made strategies and characterize specific immune cell populations.
Contributions to the MHH research focus "Oncology"
Modification of MSCs by sarcomas. In recent years, we have received a number of bone marrow samples from patients diagnosed with sarcoma from the Orthopaedic Clinical Department. We isolated MSCs from these samples and characterized their RNA profile. We found changes that differentiate the cells of sarcoma patients from those of patients without a tumor diagnosis. In addition, each sarcoma may have specific alterations. In the future, we would like to characterize selected genes functionally and extend the analysis to other cell populations.
Selected publications of the last 5 years (bold print: members of the Hoffmann group)
*: equivalent contributions, #: correspondence
Friese, N., Gierschner, M.B., Schadzek, P., Roger, Y., Hoffmann, A. (2020):
Regeneration of Damaged Tendon-Bone Junctions (Entheses): TAK1 as a Potential Node Factor.
Int J Mol Sci;21:E5177.
Lavrentieva, A.*, Hoffmann, A.*, Lee-Thedieck, C.* (2020):
Limited Potential or Unfavorable Manipulations? Strategies Toward Efficient Mesenchymal Stem/Stromal Cell Applications.
Front Cell Dev Biol;8:316
Roger, Y., Sydow, S., Burmeister, L., Menzel, H., Hoffmann, A.# (2020):
Sustained release of TGF-β3 from polysaccharide nanoparticles induces chondrogenic differentiation of human mesenchymal stromal cells.
Colloids Surf B Biointerfaces;189:110843
Roger, Y., Burmeister, L., Hamm, A., Elger, K., Dittrich-Breiholz, O., Flörkemeier, T.*, Hoffmann, A.* # (2020):
Heparin Anticoagulant for Human Bone Marrow Does Not Influence In Vitro Performance of Human Mesenchymal Stromal Cells.
Cells;9(7):E1580
Winkel, A., Jaimes, Y., Melzer, C., Dillschneider, P., Hartwig, H., Stiesch, M., von der Ohe, J., Strauss, S., Vogt, P.M., Hamm, A., Burmeister, L., Roger, Y., Elger, K., Flörkemeier, T., Weissinger, E.M., Pogozhykh, O., Müller, T., Selich, A., Rothe, M., Petri, S., Köhl, U., Hass, R., Hoffmann, A.# (2020):
Cell culture media notably influence properties of human mesenchymal stroma/stem-like cells from different tissues.
Cytotherapy;22:653-668
Gniesmer, S., Brehm, R., Hoffmann, A., de Cassan, D., Menzel, H., Hoheisel, A.-L., Glasmacher, B., Willbold, E., Reifenrath, J., Ludwig, N., Zimmerer, R., Tavassol, F., Gellrich, N.-C., Kampmann, A. (2020):
Vascularization and biocompatibility of poly(ε-caprolactone) fiber mats for rotator cuff tear repair.
PLoS One;15(1):e0227563.
Schwieger, J., Hamm, A., Gepp, M.M., Schulz, A., Hoffmann, A., Lenarz, T., Scheper, V. (2020):
Alginate-encapsulated Brain Derived Neurotrophic Factor-overexpressing mesenchymal stem cells are a promising drug delivery system for protection of auditory neurons.
J Tissue Eng;11:1-15
De Cassan, D., Becker, A., Glasmacher, B., Roger, Y., Hoffmann, A., Gengenbach, T.R., Easton, C. D., Hänsch, R., Menzel, H. (2020):
Blending chitosan-g-poly(caprolactone) with poly(caprolactone) by electrospinning to produce functional fiber mats for tissue engineering applications.
J Appl Polym Sci; DOI: 10.1002/APP.48650
Willbold, E.; Wellmann, M. Welke, B., Angrisani, N., Gniesmer, S., Kampmann, A., Hoffmann, A., De Cassan, D., Menzel, H., Hoheisel, A.-L., Glasmacher, B., Reifenrath, J. (2020):
Possibilities and limitations of electrospun chitosan-coated polycaprolactone grafts for rotator cuff tear repair
J Tissue Eng Regen Med;14(1):186-197
Scheper, V., Schwieger, J., Hamm, A., Lenarz, T., Hoffmann, A. (2019):
BDNF-overexpressing humen mesenchymal stem cells mediate increased neuronal protection in vitro.
J Neurosci Res;97(11):1414-1429
Weist, R., Flörkemeier, T., Roger, Y., Noack, S., Franke, A., Schwanke, K., Zweigerdt, R., Martin, U., Willbold E.*, Hoffmann, A.* # (2018):
Differential expression of cholinergic system components in human induced pluripotent stem cells, bone marrow-derived multipotent stromal cells, and induced pluripotent stem cell-derived multipotent stromal cells.
Stem Cells Dev;27;166-183
Hoffmann, A., Floerkemeier, T., Melzer, C., Hass, R. (2017):
Comparison of in vitro-cultivation of human mesenchymal stroma/stem cells derived from bone marrow and umbilical cord.
J Tissue Eng Regen Med; 11(9):2565-2581
Extras:
Prof. Dr. Dr. Andrea Hoffmann: Spokesperson of the DFG-FOR 2180, member of the scientific advisory board of the "Rostock Centre for Mesenchymal Stroma and Stem Cells". Member of the scientific advisory board of the "Rostock Center for Interdisciplinary Implant Research" ROCINI, member of AcademiaNet.
Teaching:
"Adult stem cells in regenerative medicine" (lecture)
"Development of cell therapeutics: Experimental applications and clinical use of adult stem cells" (lecture series)
"Introduction to animal cell culture technology" (internship at NIFE)
Teaching award 2021 for Prof. Dr. Andrea Hoffmann