Cytoskeleton in Human Diseases

Dr. Johannes Greve
Medizinische Hochschule Hannover
Gebäude I4, Ebene S0
Carl-Neuberg-Str. 1
30625 Hannover
Tel: +49-511-532-3706
Mail: greve.johannes@mh-hannover.de
Research Focus
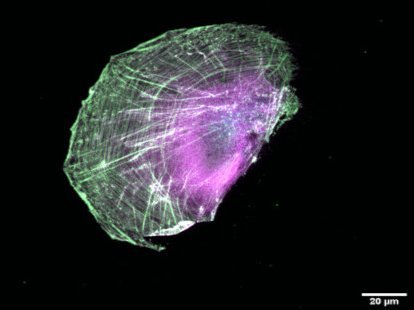
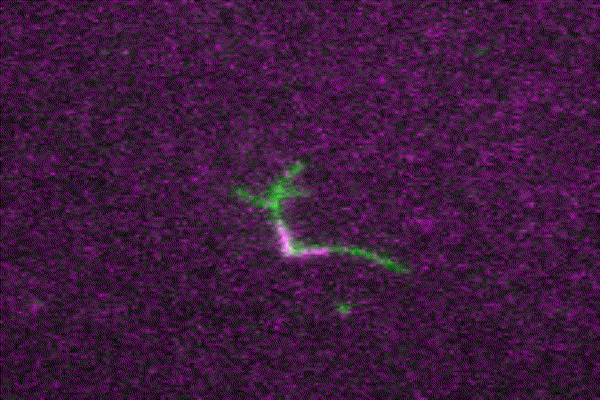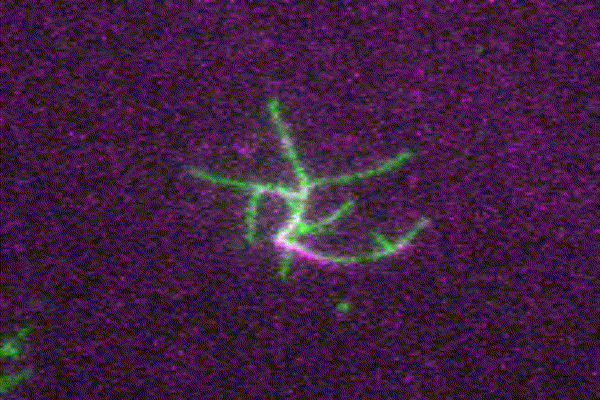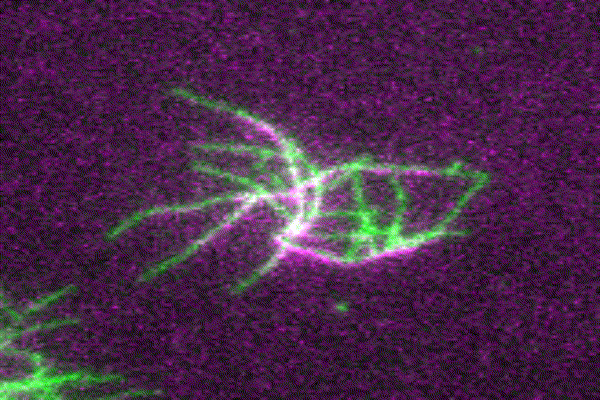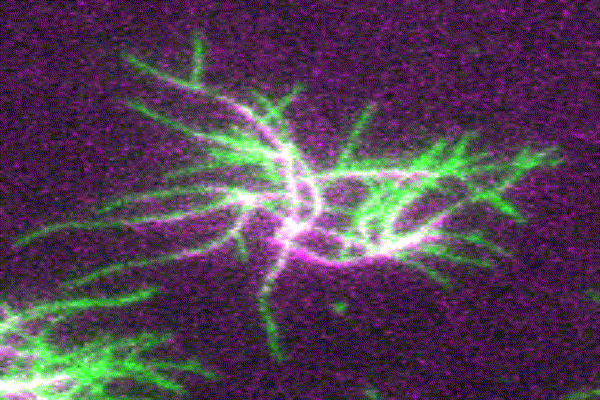
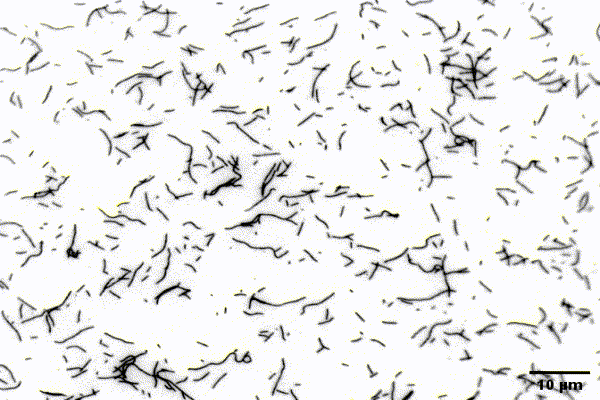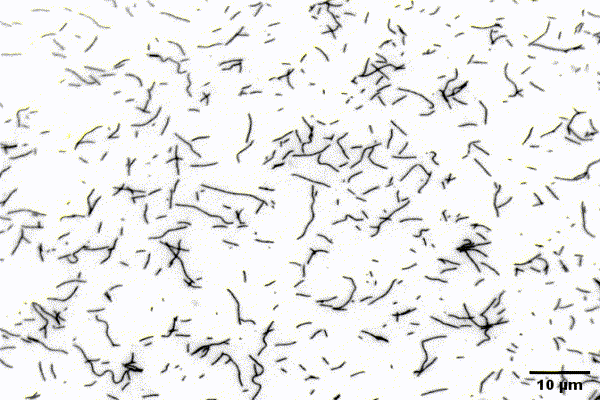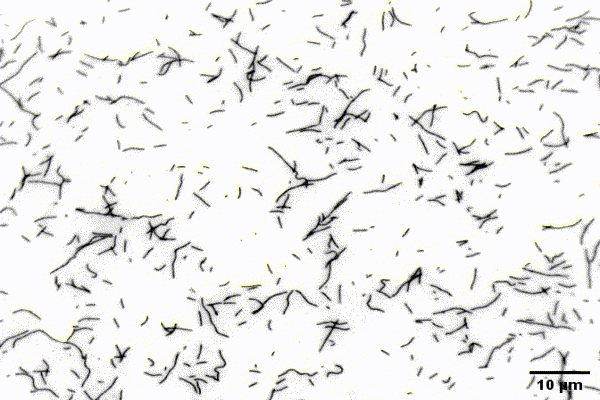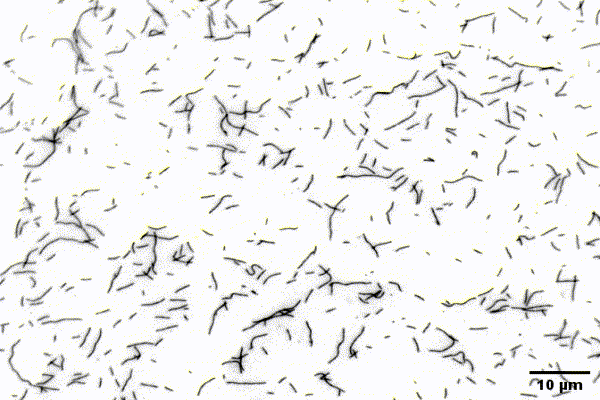
The ubiquitous actin isoforms cytoskeletal β- and γ-actin, encoded by ACTB and ACTG1, are the major components constituting the actin cytoskeleton in non-muscle cells. Essential cellular processes such as cell migration, adhesion, division, and signal transduction depend on an intact actin cytoskeleton and a robust remodelling of cytoskeletal actin structures, which enables cells to respond to intra- and extracellular stimuli by conferring cytoskeletal plasticity. The remodelling of the actin cytoskeleton, involving the assembly and disassembly of actin filaments and higher-order F-actin structures, is tightly regulated by multiple G- and F-actin binding proteins (ABPs). This regulation enables spatio-temporal control of cellular actin dynamics and drives functional compartmentalization of the actin cytoskeleton.
Several human diseases are directly associated with impaired cytoskeletal actin dynamics. Non-muscle actinopathies are mainly caused by single-point missense mutations in ACTB and ACTG1, resulting in a wide range of clinical phenotypes. Most patients present with brain malformations and neuronal migration defects, resulting in neurodevelopmental disorders and intellectual disabilities. Non-muscle myosinopathies are predominantly caused by single-point missense mutations in the actin-associated motor protein non-muscle myosin 2, encoded by MYH9. MYH9-related disorders manifest as a complex syndrome that includes thrombocytopenia and bleeding disorders. The exact genotype-phenotype correlation in actinopathies and myosinopathies is still unclear.
Our main research focus is to unravel the perturbed molecular mechanisms in actinopathies and myosinopathies by means of structure-guided biochemical and biophysical approaches. This includes, but is not limited to, in vitro reconstitution and analysis of cytoskeletal actin dynamics using TIRFM-based assays, as well as steady-state and transient kinetic approaches. This approach is complemented by in vitro and in silico structural studies of the relevant actin-ABP complexes using X-ray crystallography and molecular dynamics simulations in collaboration with the group of Prof. Dr. Dietmar Manstein.
We are always looking for motivated students interested in working on one of our current projects (see current projects). We currently offer bachelor and master thesis projects as well as laboratory internships. If you are interested, please send an informal enquiry to greve.johannes@mh-hannover.de.