Expanded Diagnostic Criteria and Phenotypic Classification Related Disease
Panel Members:
Joseph Berger (Perelman School of Medicine, University of Pennsylvania, Philadelphia, USA)
David Clifford (Washington University in St Louis, St. Louis, USA)
Irene Cortese (NINDS, NIH, Bethesda, USA), Co-Chair
Benjamin Ineichen (University Hospital Zürich, Zürich, Switzerland)
Igor Koralnik (Northwestern University Feinberg School of Medicine, Chicago, Illinois, USA)
Eugene Major (NINDS, NIH, Bethesda, USA)
Roland Martin (University Hospital Zürich, Zürich, Switzerland)
Imke Metz (University Hospital of Göttingen, Göttingen, Germany)
Sue Morgello (Mount Sinai Medical Center, New York, USA)
Avindra Nath (NINDS, NIH, Bethesda, USA)
Daniel S. Reich (NINDS, NIH, Bethesda, USA)
Clemens Warnke (University Hospital Cologne, Cologne, Germany)
Mike P. Wattjes (Hannover Medical School, Hannover, Germany), Co-Chair
Martijn Wijburg (Amsterdam University Medical Center, Amsterdam, The Netherlands)
Tarek Yousry (UCL, London, UK)
Corresponding author(s):
Irene Cortese, corteseir@ninds.nih.gov
Mike P. Wattjes, wattjes.mike@mh-hannover.de
Study Funding:
This work is partly supported by the NINDS intramural research program.
INTRODUCTION / RATIONALE
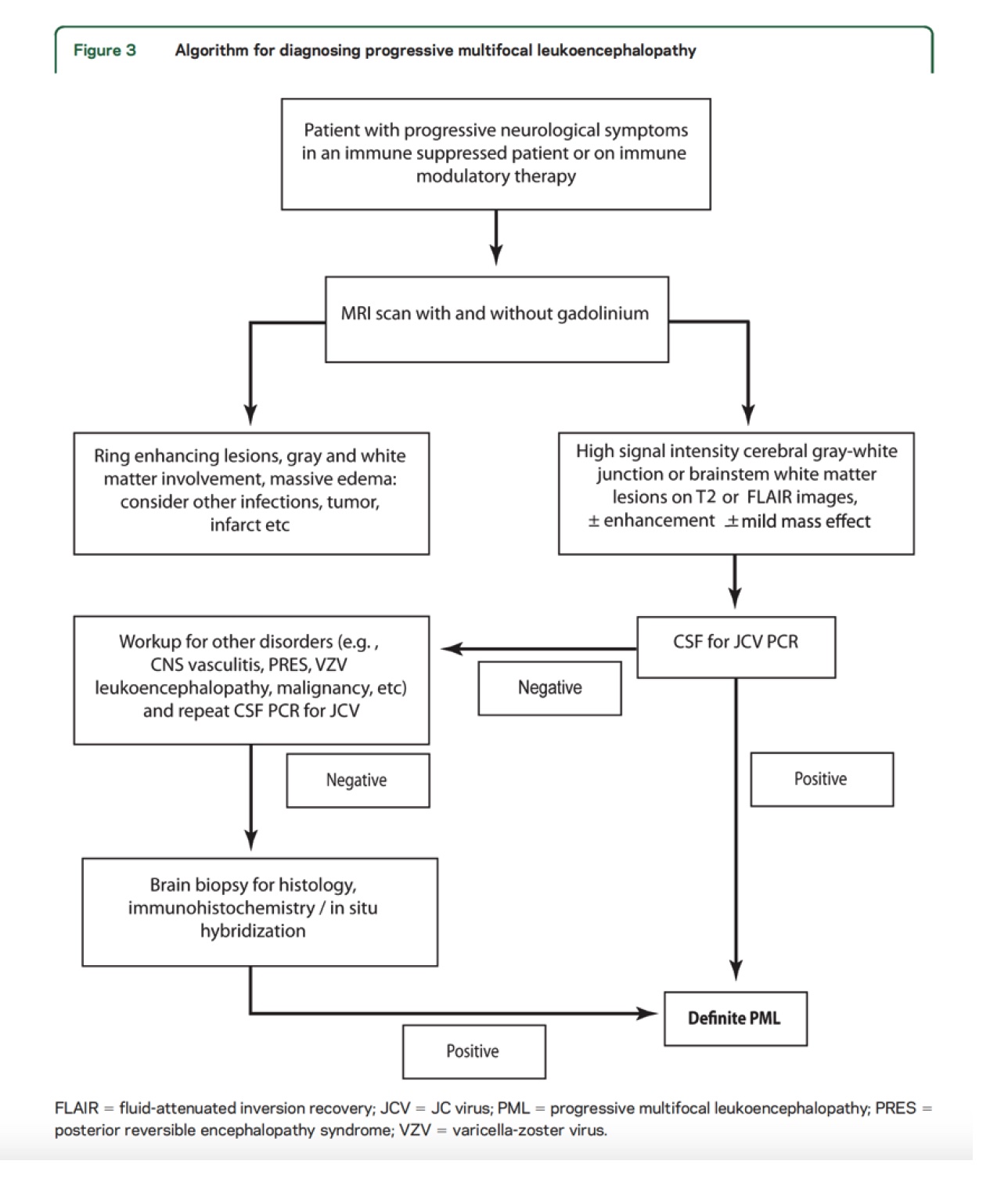
In 2013 the AAN consensus criteria for diagnosis of PML were published. This was a landmark achievement that established two approaches for diagnosis: the first based on characteristic histopathological features coupled with evidence of presence of the JCV; the second, by the presence of classic radiographic and clinical features coupled with positive CSF JCV PCR.
PML remains a feared complication of immune suppression with no available cure. Over the last decade there have been considerable advances in mapping the clinical spectrum of JCV-related disease. Further, the development of robust pharmacovigilance programs for patients at highest risk for PML have taught to detect the earliest, even preclinical stages of disease. Taken together, these urge a review and expansion of current diagnostic criteria with development of case definitions that encompass the full expression of this infection and an update of the current diagnostic algorithm so as to support best patient management, optimize outcomes and reduce practice variation. This guideline revision will further help identify research priorities on the basis of gaps in the current literature.
Overall Project Aims
-
develop consensus definitions of terms used in the context of PML diagnosis, including asymptomatic PML, classic PML, inflammatory PML, and PML-IRIS
-
develop consensus case definitions for other JC virus related diseases including granule cell neuronopathy, JC virus encephalopathy, and JC virus meningitis.
-
review, update and expand 2013 PML consensus diagnostic criteria
PICO
Patient, Population, or Problem: Patients with PML/JCV-related disease
Intervention, Prognostic Factor, or Exposure: Imaging, clinical, neuropathological and other biomarkers aiding in PML diagnosis.
Comparison or Intervention: N/A
Outcome you would like to measure or achieve: Reviewing/expanding current diagnostic criteria with development of case definitions and update current diagnostic algorithm so as to support best patient management, optimize outcomes and reduce practice variation.
Analytic Framework for 2013 Consensus Criteria
METHODS
This project will be developed in accordance with the processes described in the 2017 edition of the AAN clinical practice guideline development process manual.
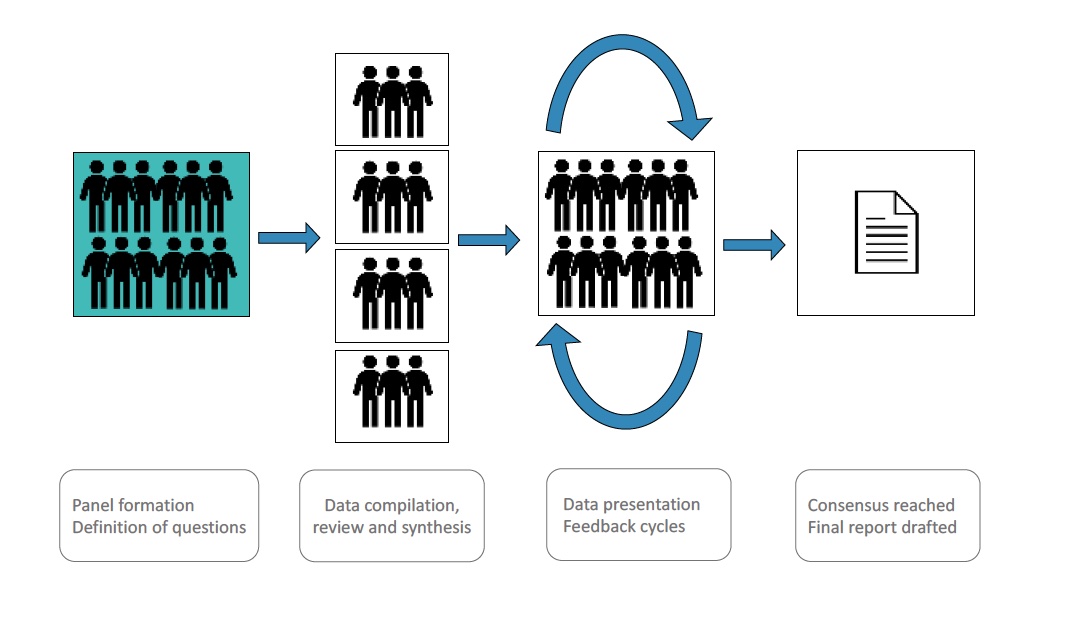
An international, multidisciplinary panel of investigators with expertise in virology, immunology, clinical neurology, neuroradiology, and histopathology was convened.
Four working groups (WG) were formed: clinical phenotypes WG, laboratory biomarkers WG, imaging biomarker WG and histopathology WG.
Study protocol will be posted for public comment prior to finalization.
In collaboration with the NIH research librarian, each WG will conduct a systematic review (SR) of the literature from (2012-2022), as detailed below. Further, all manuscripts used as data sources for development of the 2013 consensus diagnostic criteria will be reviewed and re-evaluated.
The SR will inform consensus recommendations.
Clinical questions for the systematic review
- Are there clinical phenotypes that strongly suggest PML and mandate clinicians to explore the diagnosis?
- What are the strengths and weaknesses (gaps) of the current criteria with respect to each WG area?
- Are there additional histopathological features that should be integrated into the current diagnostic criteria?
- Can imaging features of PML/JCV-related disease be specifically defined so as to facilitate early diagnosis? Enhance diagnostic specificity? Identify active inflammation?
- Are there any laboratory biomarkers that, in appropriate clinical circumstances, can be used as a surrogate for direct viral detection for diagnostic purposes? What are those clinical circumstances?
- Can discrete clinical phenotypes be identified that broaden the spectrum of JCV-related disease or are relevant to medical decision-making?
- How does each diagnostic criterion contribute to level of diagnostic certainty? If criteria are revised, how does this impact diagnostic algorithm? (Berger et al, 2013; Appendix 1)
Eligibility criteria for the systematic review
Inclusion
- Condition: progressive multifocal leukoencephalopathy (PML) and JCV-related disease
- WG outcome of interest reported: clinical description, description of imaging, laboratory assessment or histopathological examination
- Publication type: case reports, case series, original research
- Study design: any study design
- Language: English
Exclusion
- Condition: Not progressive multifocal leukoencephalopathy (PML) or JCV-related disease
- Outcomes of interest not reported
- Publication type: meta-analyses, dissertations, books, book chapters, letters, editorials, commentary, conference abstracts. Reviews including systematic reviews will also be excluded but will be retained as potential sources for additional references.
- Language: Not in English
- No abstract available
- Animal study
- Studies with serious methodological concerns (see Critical Appraisal section)
Information sources for the systematic review
The following citation and abstract databases will be searched by a biomedical librarian. Citations will be managed using EndNote X9.
- PubMed/MEDLINE (NLM)
- Embase (Elsevier)
- Scopus (Elsevier)
Search Strategy for the systematic review
Specific search terms used by biomedical librarian are:
"Leukoencephalopathy, Progressive Multifocal"[Mesh] OR “progressive multifocal leukoencephalopat*”[tiab] OR “progressive multifocal leukoencephalitis”[tiab] OR “PML Iris”[tiab] OR “inflammatory PML”[tiab] OR ((“immune reconstitution inflammatory syndrome*”[tiab] OR IRIS[tiab]) AND (PML[tiab] OR "Leukoencephalopathy, Progressive Multifocal"[Mesh] OR “progressive multifocal leukoencephalopat*”[tiab] OR “progressive multifocal leukoencephalitis”[tiab])) OR “granule cell neuronopathy”[tiab] OR “John Cunningham virus”[tiab] OR “JC virus”[tiab] OR “JC viruses”[tiab] OR “JCV encephalopathy”[tiab] OR “JC human polyomavirus”[tiab] OR “JC papova virus”[tiab] OR “JC papovavirus”[tiab] OR “JC polyoma virus”[tiab] OR “JC polyomavirus”[tiab] OR "JC Virus"[Mesh]) AND English[lang] AND ("2012/01/01"[Date - Publication] : "3000"[Date - Publication]) NOT (letter[ptyp] OR editorial[ptyp] OR comment[ptyp] OR news[ptyp] OR "Congress"[Publication Type] OR "Consensus Development Conference"[Publication Type] OR editorial[tiab] OR commentary[tiab] OR “conference abstract*”[tiab] OR “conference proceeding*”[tiab] OR “retracted publication”[ptyp] OR “retraction of publication”[ptyp] OR “retraction of publication”[tiab] OR “retraction notice”[ti] OR “retracted publication”[tiab] OR "Published Erratum"[Publication Type] OR corrigenda[tiab] OR corrigendum[tiab] OR errata[tiab] OR erratum[tiab] OR protocol[ti] OR protocols[ti] OR "Review"[Publication Type] OR "Systematic Review"[Publication Type] OR review[tiab] OR "meta analysis"[tiab] OR metanalysis[tiab]) NOT ("Animals"[Mesh] NOT ("Animals"[Mesh] AND "Humans"[Mesh])) NOT (mice[tiab] OR mouse[tiab] OR rat[tiab] OR rats[tiab] OR rodent*[tiab] OR dog[tiab] OR dogs[tiab] OR pig[tiab] OR pigs[tiab] OR piglet*[tiab] OR swine[tiab] OR porcine*[tiab] OR animal*[tiab] OR dogs[mesh] OR swine[mesh] OR rodentia[mesh])
The searches will be limited to the following parameters:
- Publication date: 2012-2022
- Language: English
Study records
Covidence platform (www.covidence.org) will be used for screening, full text review and data extraction.
Selection process for the systematic review
Each publication will first be screened by review of title and abstract using the established eligibility criteria by two study team members independently and in parallel. Discrepancies will be resolved by consensus.
The full text of selected articles will be downloaded as a PDF and uploaded to Covidence. The full text review for eligibility will be done by two study team members independently and in parallel using the established eligibility criteria. Discrepancies will be resolved by consensus.
Systematic review data collection process
Data extraction from each article will be performed by two study team members independently. The extracted data will be reviewed by the WG members who in discussion with the two data collectors will resolve any identified discrepancies, questions, or problems. Data Extraction forms are attached in Appendix 2. Extracted data will be summarized in Data Evidence Tables.
Critical Appraisal of included articles
Critical appraisal of each manuscript will be performed by the study team members extracting data; assessments will be collected in parallel to data extraction. Specifically, generalizability, quality of evidence, and risk of bias will be assessed and rated by two independent reviewers for each WG.
Based on a preliminary assessment of full texts, a considerable proportion of eligible studies is expected to be case reports/series. Thus, we will deploy the following risk of bias assessment tools:
-
For cohort and randomized controlled trials: 4-tiered classification of evidence (Class I-IV studies) will be used to classify the study relative to the study’s objectives; RCT and cohort studies will be included in this SR even if they only indirectly inform the questions asked by this panel.
-
For case reports: The Joanna Briggs Institute Critical Appraisal Tool for case reports
-
For case series: The Joanna Briggs Institute Critical Appraisal Tool for case series
-
Tools will be slightly modified to match the scope of our systematic review (Appendix 3).
Additionally, each WG will include specific data extraction items to assess robustness and appropriateness of methods used in each study:
- Clinical WG: Is clinical description sufficient? Is JCV-related disease sufficiently likely based on the reported data?
- Biomarker WG: Are methodological details about biomarker quantification sufficient? Is JCV-related disease sufficiently likely based on the reported data?
- Histopathology WG: Are the staining protocols appropriate? Are the provided histological images of high quality? How certain is PML diagnosis based on the reported data?
- Imaging WG: Is the clinical description sufficient? Are methodological details about MRI methods sufficient? Is JCV-related disease sufficiently likely based on the reported data?
Studies found to have significant risk of bias or serious methodological concerns, will be excluded from the SR.
Data Analysis
Data collected from SR will be analyzed in a qualitative manner and summarized in evidence tables in accordance with AAN Clinical Practice Guideline Process Manual.
Developing conclusions, case definitions and crafting recommendations
Modified Delphi process will be used to arrive at consensus recommendations for revision and expansion of current diagnostic criteria and to establish case definitions for inflammation in PML (classic PML, inflammatory PML, PML-IRIS) and clinical variants of JCV-related disease (asymptomatic PML, GCN, JCV meningitis, JCV encephalopathy).