Funded with 8 million euros: The MHH is leading the RESOLVE research network, which is on the trail of the "gold standard" in AML and CLL treatment
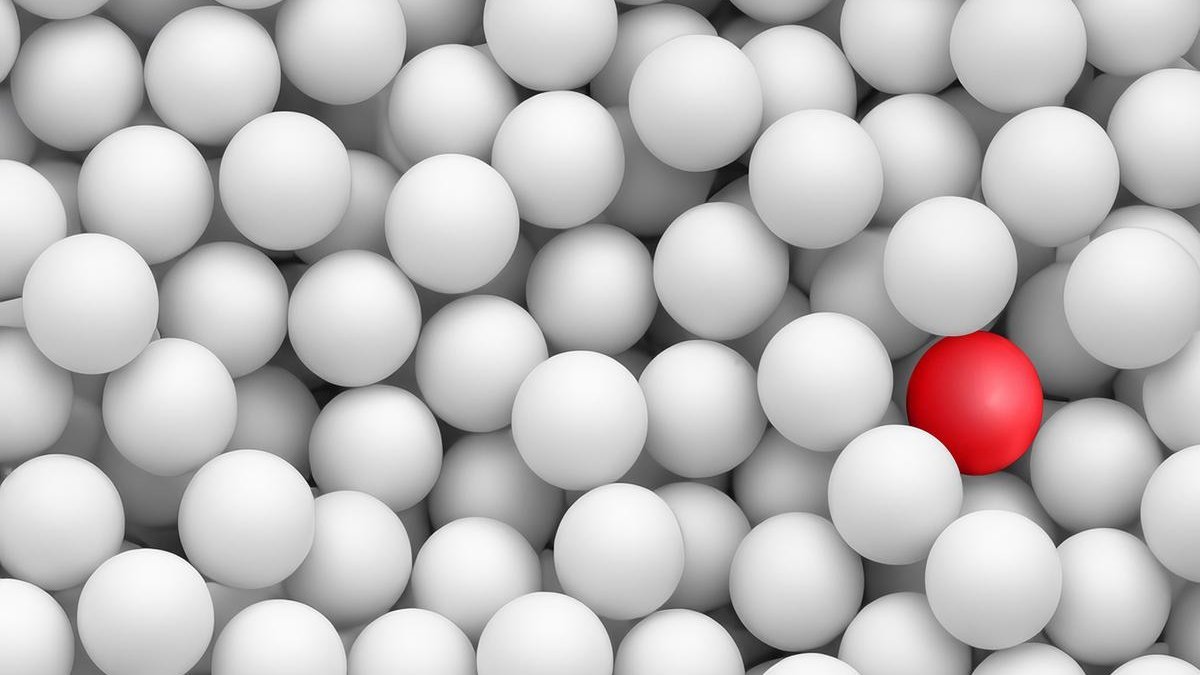
Like searching for a single red ball: flow cytometry can be used to detect hidden blood cancer cells and thus determine the risk of a relapse of the disease. Copyright: MHH/Photoshop-KI generated
Acute myeloid leukaemia (AML) and chronic lymphocytic leukaemia (CLL) are among the most common forms of blood cancer in adults. Depending on the stage of the disease, patients receive chemotherapy, immunotherapy or a stem cell transplant. The so-called measurable residual disease (MRD) can be used to determine whether the leukaemia is responding very well to the therapy early on in the course of treatment. It is present when very sensitive measurement methods such as flow cytometry still detect leukaemia cells in the body that cannot be detected with a light microscope. Although MRD has a high predictive value for further treatment, it has not yet been sufficiently scientifically tested to serve as a binding guideline for an individual treatment recommendation. The RESOLVE research network, led by Professor Dr Michael Heuser, Senior Consultant at the Department of Haematology, Haemostaseology, Oncology and Stem Cell Transplantation at Hannover Medical School (MHH), is now seeking to clarify this.
The consortium of 21 institutions in eight European countries intends to utilise several existing expert networks and partnerships with patient participation for the study. "If we confirm MRD as a treatment-guiding biomarker, it can be used to guide the treatment of AML and CLL across Europe in the future," says Professor Heuser. This could mean that more intensive therapies such as stem cell transplants could be dispensed with or shorter treatment times could be sufficient. This could improve the quality of life for patients and reduce treatment costs at the same time. The European Union is funding the project with a total of eight million euros over five years. Of this, 2.2 million euros will go to the MHH.
Equal chances of recovery with fewer side effects
With flow cytometry, several hundred thousand cells can be examined simultaneously within a short period of time using a high-throughput procedure, allowing individual leukaemia cells to be detected in the midst of healthy blood cells. "It's like looking for a needle in a haystack or a single red ball in a pool full of white balls," explains Professor Heuser. The aim of the therapy, so to speak, is to ensure that there are no more red balls to be found, i.e. that there are no more blood cancer cells in the body. "Based on this, we are investigating whether less medication or shorter treatment offers MRD-negative patients the same chances of recovery - with fewer side effects." In some countries, MRD measurement is already being used to individually assess the risk of a relapse after successful cancer treatment and to precisely adapt further therapy on a personalised basis. The researchers now want to scrutinise the measurement method in a large clinical study with 60 participating clinics and develop binding standards to ensure that the same method is used everywhere in future. "In this way, we want to prove the clinical, personal and social effects of MRD-guided therapy," emphasises the haematologist.
Establishing MRD assessment as the "gold standard"
The MRD assessment based on the flow cytometry examination should then be introduced as quickly as possible throughout Europe as the "gold standard" for the individual management of leukaemia treatment. To this end, the researchers are using existing infrastructures for laboratories and clinics as well as expertise from the European research networks for CLL "ERIC" and AML "ELN-DAVID", which Professor Heuser chairs. Representatives from politics, patient associations, nursing, social sciences and health economics will also be involved. "We want to create a platform for routine clinical practice that helps medical staff to determine the individual risk of relapse for AML and CLL patients and avoid overtreatment," emphasises the project manager. The aim is to give all patients throughout Europe access to this test in the national healthcare systems.
The RESOLVE project (Residual disease assessment in hematologic malignancies to improve patient-relevant outcomes across Europe) is part of the "EU Mission on Cancer" funding line, with which the European Union supports projects that aim to improve the lives of more than three million people with cancer by 2030. In addition to the Department of Haematology, Haemostaseology, Oncology and Stem Cell Transplantation, the Comprehensive Cancer Center (CCC) and the Centre for Clinical Studies (ZKS) at the MHH, which is the legally responsible sponsor representative and is responsible for initiation, management and quality assurance, 20 other institutions and 60 hospitals from France, Germany, Greece, Israel, Italy, Poland, Spain, and The Netherlands are involved.
►More information on the RESOLVE project can be found here.
Text: Kirsten Pötzke