Cytostatics are a group of chemically different substances that prevent cell division by interfering with the metabolism of the cells. Cytostatics are used in the treatment of many different diseases, including oncology. In the MHH pharmacy, 120 individually dosed cytostatic solutions are prepared every day using aseptic working techniques and taking into account optimal personal protection. These are prepared in specially equipped and qualified clean rooms by our trained specialist staff. Through intensive interdisciplinary cooperation with the medical staff, each prescription is prepared on the basis of patient data and checked for plausibility according to clinical-pharmaceutical aspects before production, thus ensuring optimum therapeutic safety. Advising physicians on all questions relating to cytostatics, their dosage, side effects and interactions is a top priority for the pharmacy.
Contact person:
Jesco Stember
Phone: (0511) 532-3327
Email: Stember.Jesco@mh-hannover.de


Parenteral nutrition is the supply of nutrients by "bypassing the gastrointestinal tract". This is necessary if a patient is unable to consume food by enteral means (in the "normal way" or via a tube) for a longer period of time, e.g. in the case of post-operative conditions, diseases of the abdominal organs or if there are significant digestive or absorption disorders.
In the mixed infusion unit, individually composed nutritional solutions are prepared daily for up to 20 patients for neonatology and for the entire pediatric clinic, as well as for adult patients. If the patient's requirements cannot be covered by an industrially available bag, the parenteral nutrition must be prepared individually. For each of these patients, an individual supply of electrolytes, trace elements, amino acids, carbohydrates, fat and vitamins is required, which is adjusted daily to the respective needs.
The medical prescriptions are subjected to a plausibility check in the pharmacy according to clinical-pharmaceutical aspects to increase drug safety. Preparation then takes place centrally in the hospital pharmacy under validated, strictly aseptic conditions on a ceiling laminar air flow using a pump system.
Between 10,000-12,000 mixed infusion bags are produced annually for the entire Clinical Department.
Contact person:
Hassan Darkhabani
Phone: (0511) 532-3328
Email: Darkhabani.Hassan@mh-hannover.de

"Everything that must be sterile", i.e. free from microorganisms capable of reproduction such as bacteria or fungi - from eye drops to injection solutions and infusion solutions. In the sterile department of the MHH Central Pharmacy, we produce medicines for the Clinical Department that are not offered by the pharmaceutical industry.
Of course, all medicines are generally produced under hygienic aspects. However, whether a medicine must be sterile, i.e. absolutely germ-free, depends on the application method, i.e. how it is administered.
If the medicine is administered externally (creams, ointments, tinctures, plasters, ...), the body has the skin and its sebum or sweat as a protective barrier. When taken orally (drops, juices, tablets, ...), for example, the mucous membranes of our digestive tract, stomach acid and the constant removal during digestion protect against the penetration of germs.
However, if a drug is administered invasively, i.e. directly into the bloodstream (injections, infusions, injections, etc.), the physiological barriers are bypassed and the penetration of germs could have fatal consequences. For this reason, invasively administered medicines such as infusions or injection solutions must be absolutely sterile. The same applies to preparations for use on the eye.
Production takes place according to recognized pharmaceutical rules and with special attention to hygienic aspects in our new clean rooms (commissioned in 2017).
In order to ensure that our medicines are of a consistently high quality, our activities include a large number of quality controls in addition to the actual manufacturing process.
Contact person:
Jessica Gunia
Phone: (0511) 532-5604
Email: Gunia.Jessica@mh-hannover.de


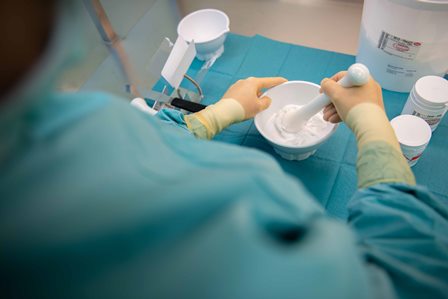
If medicines are required that are not produced industrially, they must be prepared on site in the pharmacy by pharmaceutical staff. This applies, for example, to medicines whose shelf life is too short for industrial production, which are significantly cheaper to produce in-house or preparations with special strengths for specific patient groups. In the field of pediatrics in particular, finished medicinal products are often not available in the required dosage, meaning that lower-dose preparations in the form of capsules or juices, for example, usually have to be produced.
The Department for non-sterile production is primarily concerned with the production of ointments, creams, capsules, solutions, juices and suppositories. In the formulation department, preparations are made individually for each patient. In the case of frequently requested preparations, up to 100 ready-to-dispense preparations per day may be produced in stock in order to cover the clinic's requirements.
The same requirements are placed on the quality of the products as on industrially produced finished medicinal products. By using tested raw materials, optimizing work processes and participating in external quality inspections, we can ensure impeccable quality.
Contact person:
Gunnar Dubenhorst
Phone: (0511) 532-8615
Email: D ubenhor st.Gunnar@mh-hannover.de

The safety and efficacy of new drugs and new treatment options are tested in clinical trials under controlled conditions. If an advantage of the tested substance or form of treatment compared to the previous medical standard is shown during this test, it can be used on larger patient groups. Clinical trials are therefore essential for medical progress.
In compliance with the GCP (= Good Clinical Practice) guidelines, the pharmacy is involved in the implementation of the studies in everyday clinical practice. Our qualified specialist staff are involved in the production of patient-specific preparations, product logistics, storage of study goods including monitoring of storage conditions and documentation.
In order to prevent distortion of the results, in many studies the patient and the treating physician are not aware of the allocation to the respective therapy group. This so-called blinding of the therapy and the randomization (= random allocation) of patients and study goods are carried out by the pharmacy.
In these extensive tasks, the Central Pharmacy of the MHH draws on many years of experience in the field of clinical studies and is currently involved in the implementation of over 100 studies.
Contact person:
Nicole Andermark
Phone: (0511) 532-8638
Email:
Studien.Apotheke@mh-hannover.de
Andermar k.Nicole@mh-hannover.de