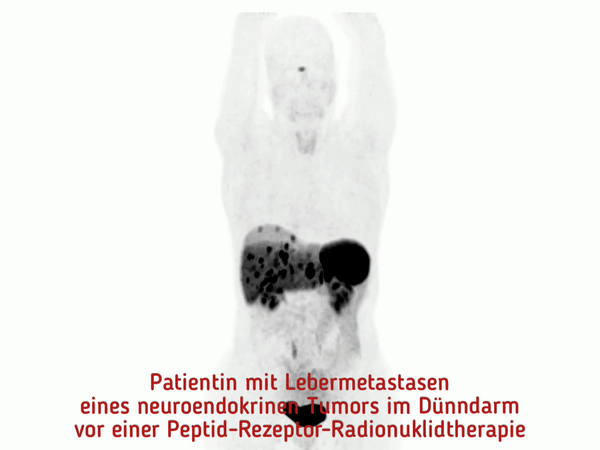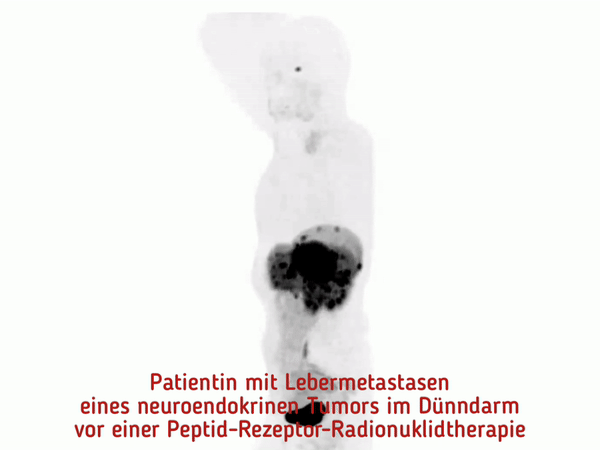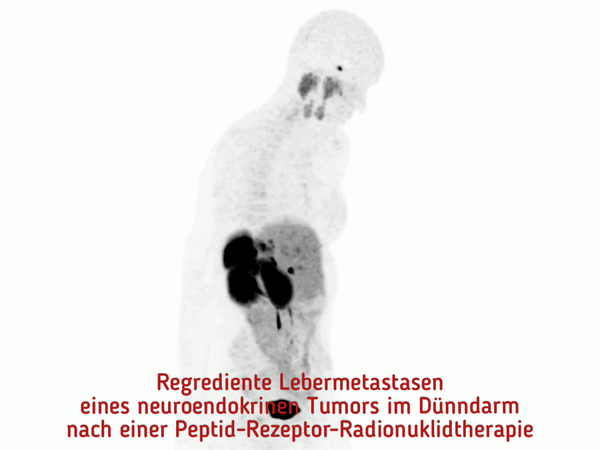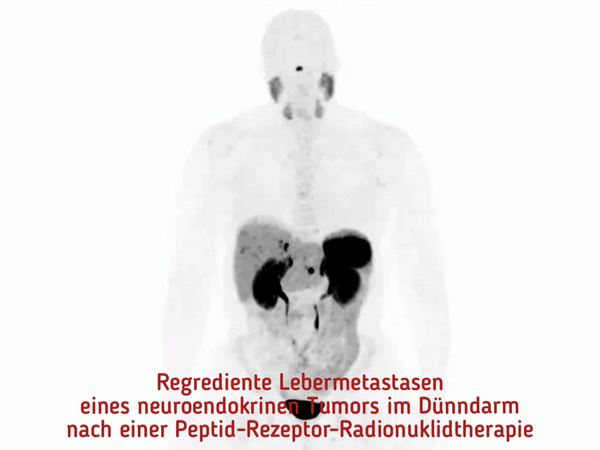
At the MHH, pioneering work was done to establish peptide receptor radionuclide therapy (PRRT). This is a new, highly effective procedure for the targeted treatment of neuroendocrine tumors.
The documents required to register for PRRT can be found here.
Neuroendocrine tumors (NET) are often slow-growing but malignant neoplasms. They often cause symptoms such as diarrhea, paroxysmal sweating (flushing) and increased blood pressure. Due to the rather slow growth and the not always typical symptoms, many patients initially only require symptomatic treatment (medication to adjust blood pressure and/or inhibit diarrhea). Causative chemotherapeutic treatment is only used at an advanced stage, as its effectiveness is limited due to the slow growth of the tumor. More effective therapies have only been available for a few years, e.g. with Sandostatin, which not only eliminates the diarrhea but also binds to the somatostatin receptors typically present in carcinoid cells, thus slowing tumor growth without causing symptoms such as nausea, vomiting and hair loss. In order to enhance this effect, it made sense to use a radioactively labeled derivative of sandostatin against NET. Various radionuclide-doped peptides have therefore been used for around 10 years.
The drug used is a ligand labeled with lutetium-177 or yttrium-90. Ligands also occur naturally; they are small components (peptides) that bind to a receptor and thus transmit a signal at the cellular level.
Once the drug is in the bloodstream, the ligand recognizes healthy as well as malignant ("cancer") cells that carry the somatostatin receptor on their surface and binds to them. The radioactive particles that are bound to the ligand kill the cells occupied by the ligand.
All treatment options are discussed in an interdisciplinary tumor board involving internists, surgeons, radiotherapists and nuclear medicine specialists before the decision to undergo PRRT in order to determine the best possible treatment strategy.
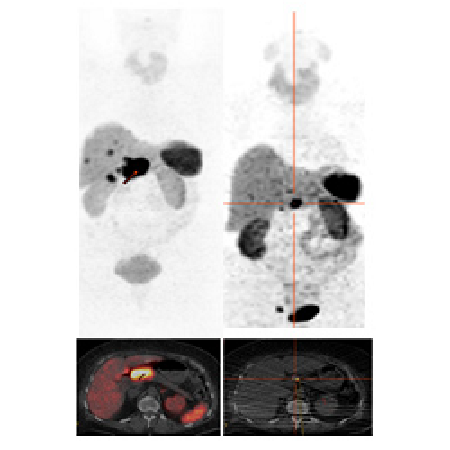
In order to avoid occupying the somatostatin receptors with the often therapeutically administered depot sandostatin (this would result in fewer binding sites for the radioactive peptide), this should be discontinued at least 6 weeks before therapy. Due to the possible kidney-damaging effect of the radiopeptide, a thorough examination of kidney function is planned before starting therapy and as a follow-up before possible further therapies. In addition to the usual laboratory tests (creatinine and urea), a tubular extraction rate of the kidneys is determined using a nuclear medicine procedure. Furthermore, image-supported diagnostics (PET/CT) are carried out before each tumor therapy. This determines how many tumor foci are present and whether they have sufficient receptor occupancy for therapy. Depending on the type of tumor and the symptoms present, further examinations such as tumor marker determination (chromogranin A) may be performed.
For the actual therapy, an indwelling venous cannula is inserted and connected to an infusion system. An amino acid solution is administered approximately 2 hours before the start of therapy to protect the kidneys and prevent excessive uptake of the radioactive peptide in the kidneys. The infusion is continued over a period of 4 hours. The radiolabeled peptide is then also administered via the indwelling cannula using a perfusor over a period of 15 minutes. Regular pulse and blood pressure checks are carried out during the therapy. According to the radiation protection guidelines, a 48-hour stay on the therapy ward is prescribed after therapy. During this period, three whole-body scintigraphies are performed after 24 hours to document the fate of the radioactive substance and to estimate the focal doses achieved (tumor/organ doses).
The side effects known to date include non-specific symptoms such as headaches and fatigue. Increased flushing symptoms are also possible, which can last for several days. Nausea and vomiting may also occur after therapy. In rare cases, tumor cell destruction leads to a very high release of hormones, which can be accompanied by circulatory and respiratory problems, headaches and neurological symptoms. In addition, blood count changes with a reduction in the number of red blood cells (erythrocytes), platelets (thrombocytes) and white blood cells (leukocytes) are possible in the medium term; regular blood count checks after therapy are therefore recommended. Due to the radiation exposure of healthy liver tissue, liver function may be impaired. For this reason, monitoring of liver parameters is also recommended. In patients with extensive liver metastasis, radioactive irradiation of the liver tissue may cause swelling of the liver with temporary stretching of the liver capsule, which may cause pain. Prophylaxis with cortisone is therefore recommended for patients with pronounced liver metastasis. Allergic reactions rarely occur when the therapeutic substance is administered. Repeated therapy can lead to a reduction in kidney function. Precautions have been taken to ensure that competent medical care is available in the event of any side effects.
The therapy can be carried out in several cycles depending on the accumulation of the radioactive substance in the tumor foci, the kidney function and the organ doses achieved up to that point (the kidney is the limiting organ), which is checked before each cycle (of approx. 3 months). After therapy, regular checks are planned with CT, renal scintigraphy and PET/CT to record the effects and side effects and to decide on possible further therapy.
Inclusion criteria
- Age between 18 and 70 years
- Neuroendocrine tumor (histologically proven) with evidence of sufficient somatostatin receptor expression in scintigraphy or PET/CT
- Tumor progression under/after standard therapy
- Written consent of the patient for therapy
Exclusion criteria
- Another malignant secondary disease
- Impaired renal function with pathological 99mTc-DTPA or 99mTC-MAG3 scintigraphy or elevated creatinine or urea levels.
- Bone marrow depression after chemotherapy
- Poorly differentiated neuroendocrine tumors with a high proliferation index
After a temporary interruption, PRRT can currently be offered again at the MHH. Our clinic works closely with the Clinical Department of Gastroenterology, Hepatology and Endocrinology and the corresponding gastroenterological tumor board to determine indications and provide optimal patient care.
" Contact information for PRRT coordination
