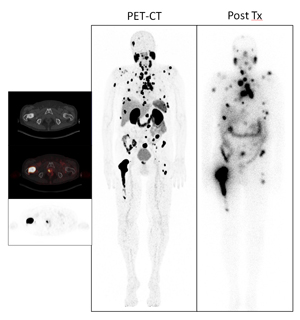
The latest form of radionuclide therapy at the MHH is PSMA ligand therapy for advanced prostate cancer.
The target group is patients with metastatic prostate cancer in whom the disease has progressed despite exhaustion of guideline-based therapy.
There is close cooperation with the urological-oncological tumor board of the MHH to determine indications and provide optimal patient care.
Before the actual therapy on ward 75, an outpatient consultation takes place. A current PSMA PET-CT and, if necessary, a kidney scintigraphy must also be available.
Suitability for therapy can be assessed using the checklist below. checklist below.
PSMA (prostate-specific membrane antigen) is a protein that is found on the surface of prostate cancer cells. In therapy with 177Lu-PSMA ligands, a substance radioactively labeled with a particle emitter is injected, which then binds specifically to tumor cells and achieves its effect there via the radiation effect. This is a therapy that is not approved as a medicinal product and is produced at the Medical University in compliance with the strictest quality requirements and under the supervision of the relevant regulatory authorities.
The therapy is palliative, i.e. a cure is unlikely to be achieved, but the aim of the therapy is to reduce the tumor mass, lower the PSA value and alleviate symptoms such as pain. Comparisons on the effectiveness of the therapy compared to established procedures are only available to a very limited extent. According to studies to date, around two thirds of treated patients benefit from this therapy (reduction in PSA levels).
The therapy is administered in hospital via a venous access, with a single dose being given ("1 cycle"). You will receive an infusion of fluid to accompany the therapy. You should drink at least 2 liters a day during the days following the therapy. You will also receive cooling compresses for your salivary glands on the day of therapy. Several cycles of 177Lu-PSMA ligand therapy can be given, usually 6 weeks between each cycle.
Since a particle emitter is administered here, the therapy is accompanied by radiation exposure. This also means that, according to the radiation protection legislation applicable in Germany, you must stay on the nuclear medicine ward for a few days, are not allowed to leave it during this time and cannot receive visitors. After discharge, blood counts and other laboratory values should be checked after two weeks and then at four-week intervals. These will be specified in the discharge letter.
Experience to date has shown that the therapy is well tolerated. However, as with any systemic therapy, side effects and complications can occur, in particular the following general and specific risks:
- in < 10% of patients, several weeks of exhaustion (fatigue symptoms), nausea
- temporary / sometimes permanent dry mouth (xerostomia) due to damage to the salivary glands (especially in the case of previous damage to the salivary glands and after several treatment cycles)
- Temporary / sometimes permanent deficiency of red and white blood cells and platelets (anemia, leukopenia, thrombocytopenia), possibly requiring transfusions
- Kidney damage up to the need for dialysis
- Allergic reactions up to life-threatening reactions
- If injected next to the vein: extensive tissue destruction with the possible need for surgical therapy/amputation (risk of extravasation including radionecrosis to loss of extremities)
- Access: local infection, nerve injury
- Principle risk of induction of secondary malignancies due to radiation exposure, but long latency period
