Research
We not only ensure the supply of diagnostic and therapeutic radiopharmaceuticals from the Clinical Department of Nuclear Medicine, but also conduct research into new diagnostic methods using PET and SPECT. Below you will find an explanation of the research areas on which the team headed by Prof. Dr. Ross is working.
MicroRNAs (miRNAs) are short (~20 nucleotides), non-coding RNAs that regulate gene expression post-transcriptionally and thus play an important role in many biological processes (e.g. apoptosis, differentiation, proliferation). Accordingly, dysregulation of individual miRNA families contributes to the development of widespread diseases (cancer, neurodegenerative and cardiovascular diseases). The miRNA level therefore represents a promising new biomarker for the pre-symptomatic diagnosis of various diseases. With the help of radioactively labeled anti-miRNA oligonucleotides, which bind to complementary miRNAs, it is possible to examine the miRNA level in vivo by PET/ SPECT.
In this project, labeling strategies for an anti-miRNA oligonucleotide with F-18 and Ga-68 will be developed. Subsequently, the radiolabeled anti-miRNAs will be evaluated in preclinical PET/CT studies in different disease models.
Cardiovascular diseases in general are the most common cause of death in Europe. The development of chronic heart failure plays an important role here, and despite significant therapeutic advances, the prognosis is still poor. It often occurs as a result of various previous disease processes, such as a heart attack, in which the occlusion of a coronary artery leads to the death of the underlying heart muscle cells with scarring. This can be triggered by cardiac cachexia, which is often neglected in everyday clinical practice, especially in the context of cancer, as the focus is on treating the underlying malignant disease. However, tumor-associated cardiac cachexia not only severely restricts patients' quality of life, but is also a decisive prognostic factor.
The aim of our research in this field is to demonstrate the pathophysiological changes in ischemic tissue. The temporal course as well as regeneration processes and the success of prophylactic strategies are to be investigated using nuclear medicine methods. Various radioactively labeled tracers are used as diagnostic markers.
The term click chemistry was defined by Sharpless et al. in 2002 and describes a series of highly efficient and fast reactions. Various other requirements such as high yields (quantitative), mild conditions, fast kinetics, biocompatible, low toxicity, etc. must be met to become a "click reaction". A well-known example is the Cu(I)-catalyzed version of the Huisgen 1,3-dipolar cycloaddition of alkynes and azides to 1,4-triazoles. This reaction provides almost quantitative yields in a short reaction time and under very mild (biocompatible) conditions. In addition, alkynes and azides are inert under most reaction conditions and do not react with most of the common functional groups.
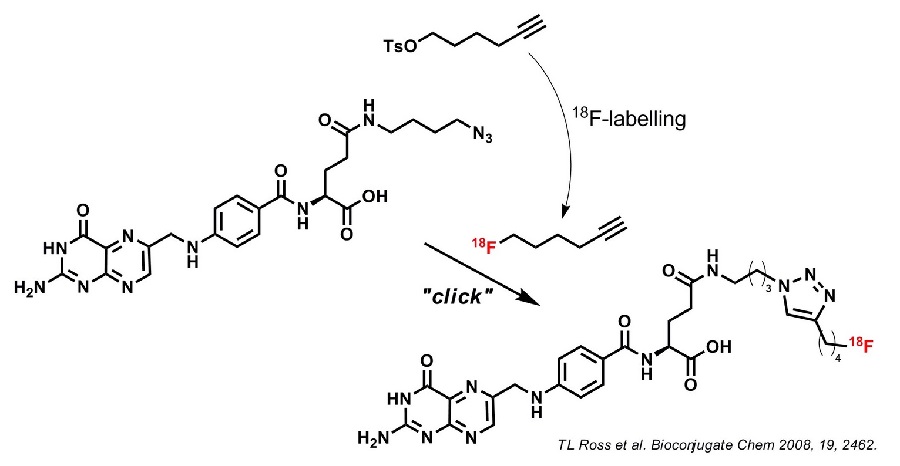
In radiopharmaceutical chemistry, we often work with complex biomolecules that are sensitive to harsh reaction conditions and also carry many functionalities. The preferred direct 18F radiolabeling of such molecules often turns out to be impossible and only indirect methods such as the use of prosthetic groups are the last resort. We use Click Chemistry for indirect 18F-labeling of complex biomolecules and structures. We are also working on the development of new "clickable" prosthetic groups for 18F labeling.
Since the above mentioned click cycloaddition is such a highly efficient and selective method to couple two molecules, we continue to use click chemistry for various assembly syntheses and linkage chemistry of complex structures and molecules.
Around 370 million people worldwide suffer from diabetes. For the future, studies predict an increase to over 500 million people by 2030. There is currently no direct diagnostic procedure available for early detection.
A basic distinction is made between type I and type II. Type I is an autoimmune disease in which the body's own insulin-producing beta cells are destroyed. In contrast, type II diabetes is a chronic metabolic disease that results in an increased blood sugar level.
The progression of diabetes is closely linked to the mass of beta cells present, especially in type I diabetes. Quantitative determination of beta cell mass in vivo is currently not possible. Non-invasive, quantitative imaging methods can close this important gap. In this project, we are developing the corresponding beta cell-specific radioligands.
The clinical picture of liver fibrosis is a chronic wound healing process in which excessive deposition of extracellular matrix (ECM, mainly type I collagen) occurs in the hepatic tissue. The permeation of plasma components from the hepatic sinusoids through the space of Disse - now filled with collagen - into the hepatocytes is thus impeded. This leads to impaired liver function, increased intrahepatic resistance (portal hypertension) and often to the development of liver cancer. The excessive deposition of ECM is caused by an imbalance between fibrogenesis and fibrolysis. If the liver is repeatedly attacked (e.g. by alcohol, toxins, hepatitis B/C, autoimmune diseases, etc.), collagen synthesis is upregulated via various mechanisms (see illustration). The collagen-producing cells are activated myofibroblasts, which can develop from hepatic stellate cells as well as from portal or perivascular fibroblasts and hepatic endothelial cells.
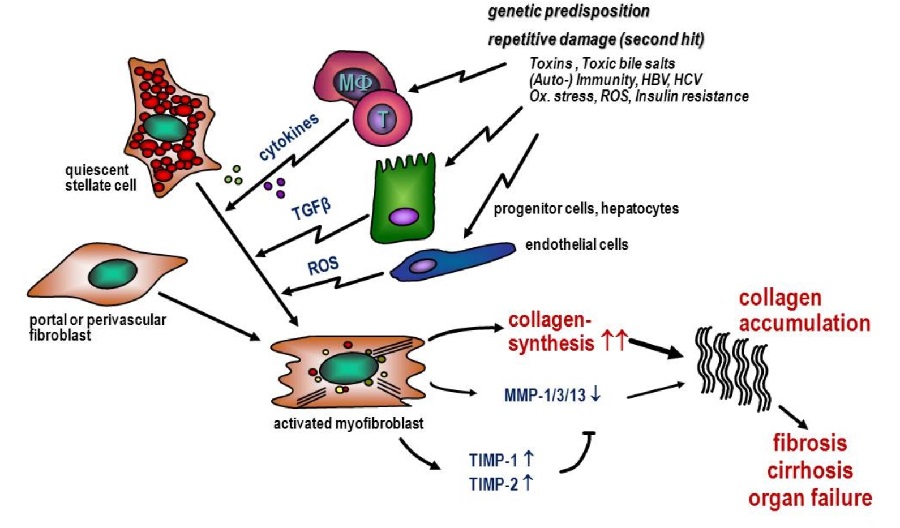
Unfortunately, the diagnosis of liver fibrosis is difficult. The gold standard is still the liver biopsy with subsequent histological examination of the tissue removed. However, even with this method, the results regarding the severity of the fibrosis vary greatly depending on where the sample is taken (representing only about 1/50,000 of the organ). This complicates both treatment in the Clinical Department and the development of new anti-fibrotic therapeutics.
Positron emission tomography (PET) is an excellent method to include the inhomogeneity of the disease within the liver in the diagnosis, as it allows the entire organ to be examined. The development of a PET tracer could therefore both significantly improve the diagnosis of liver fibrosis and, as a non-invasive method, bring great benefits for patients. Of particular interest are targets that reflect the extent of fibrogenesis, as this allows the response of a therapy to be examined at a very early stage. To this end, we are developing small molecules and peptide structures that are labeled with 18F and 68Ga and are being evaluated as potential radiopharmaceuticals for PET imaging of fibrosis and fibrogenesis. In this project we are working in close collaboration with the research group of Prof. Dr. Dr. D. Schuppan (University Medical Center Mainz)
Folic acid (FS) is a vitamin (B9) and the bioactive form of 5,6,7,8-tetrahydrofolate (THF). In the body, ingested FS is reduced by dihydrofolate reductase (DHFR) to provide the bioactive form THF. THF is essential for the one-carbon metabolism (e.g. de novo DNA synthesis) of cells. THF and FS are therefore irreplaceable for cell development and cell growth.
In general, there are two mechanisms for cellular uptake. The reduced folate carrier (RFC) is a transporter protein that transports THF directly into the cell cytosol. In contrast, the oxidized form (FS) can only be taken up by endocytosis via binding to the so-called folate receptor (FR).
In normal (healthy) tissue, FR expression (apical) is extremely limited and restricted to the kidneys, lungs, placenta and choroid plexus. In contrast, many human epithelial carcinomas such as ovarian cancer, colon cancer, breast cancer, etc. strongly overexpress FR. As a result, FS and pteroic acid are increasingly taken up by malignant cells via the FR and show a high accumulation in the tumor tissue. For this reason, radiolabeled folic acid or pteroic acid derivatives are extremely promising and ideal candidates for tumor targeting and oncological imaging
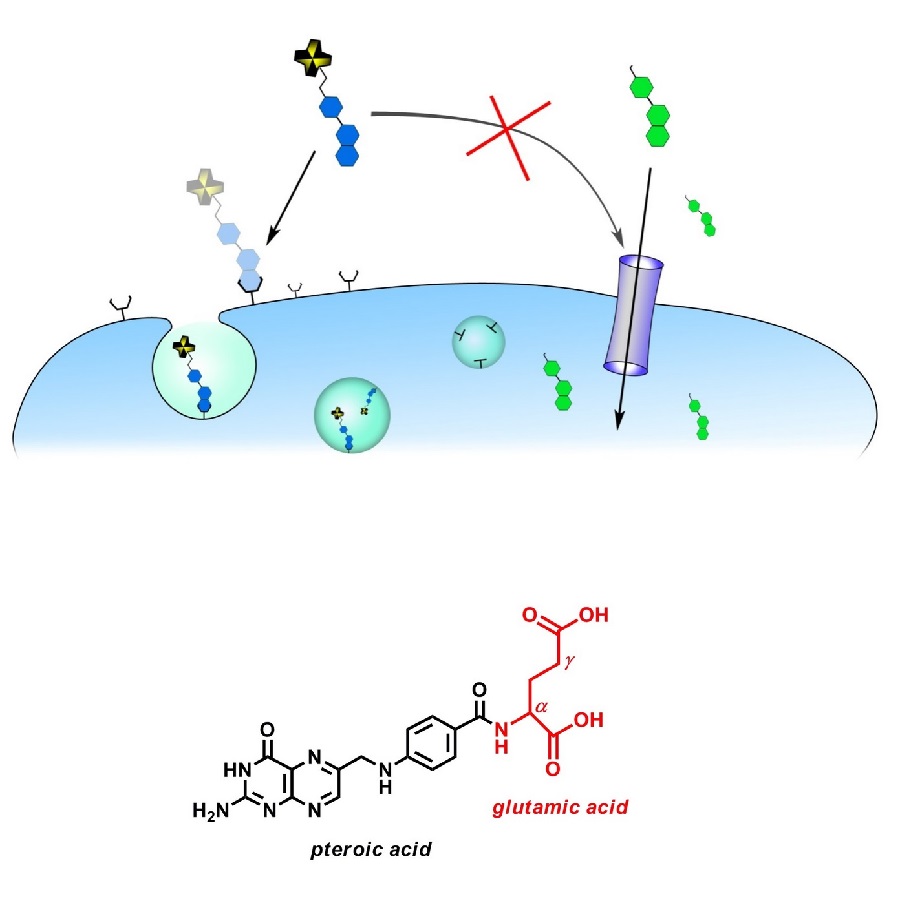
The folic acid molecule is divided into two building blocks. One subunit, the pteroic acid part, is assumed to be responsible for the high (nanomolar) affinity to the FR. The other part, the glutamic acid unit, even allows extensive chemical modifications without affecting the affinity to the FR. Consequently, the two carboxyl groups on the glutamate are available for derivatization and labeling. Since there are two carboxyl groups (α and γ), the regioisomerism cannot be ignored. Complex regioselective syntheses are necessary to avoid isomeric mixtures.
As mentioned above, folic acid is an optimal vector for tumor targeting and imaging. Although a few FS-based radiotracers for PET have already been developed, there is still a great need for FS radiotracers that are synthetically easily accessible with high radiochemical yields and show suitable pharmacokinetics. We are therefore working on the synthesis of new folic acid derivatives. To this end, we synthesize various 18F- and 68Ga-labeled folic acid-based substances and investigate their in vitro and in vivo behavior.
Although nanoparticles represent a relatively new aspect in pharmacological research and especially in routine clinical practice, similar products have been used in nuclear medicine studies for decades. These compounds became known as colloid-based radiopharmaceuticals, radiolabeled nanocolloids or simply radiocolloids. As with passive targeting mechanisms of modern nanoparticle systems, their beneficial tissue enhancement is based on their particle size and physicochemical (particle surface) properties.
At the end of the 20th century, when colloid chemistry was reborn as "nanotechnology" and its medical applications as "nanomedicine", the demands on the production of new generations of diagnostic and therapeutic drugs have increased. Molecular imaging techniques are excellent tools for the development of new, targeted drug delivery particle systems. At the same time, this approach offers the possibility to develop innovative diagnostic and/or therapeutic radiopharmaceuticals.
Compared to conventional small molecules, nanodimensional colloidal particles can transport a larger amount of radioisotopes and drugs. Through passive enrichment and molecular targeting vectors bound to their surface, nanoparticles can selectively and efficiently target diseased tissues and organs. Under ideal conditions, a nanoparticle circulates in the body for a relatively long time and can therefore continuously accumulate in the target tissue. By modifying nanoparticles, specificity, efficacy and bioavailability can be optimized, thus reducing side effects.
Together with our cooperation partners, we are tackling the following radiopharmaceutical challenges:
- Nano-radiopharmaceuticals: development of new nanoparticle-based radiopharmaceuticals for the diagnosis and therapy of tumor diseases and infections.
- Preclinical evaluations: Molecular imaging techniques are used to test new nano-radiopharmaceuticals and nanodimensional drug delivery systems for their pharmacological potential.
- Controlled Drug Release Imaging: Visualization and quantitative monitoring of controlled drug release systems that can respond to external remote stimuli.
Bacterial infections still pose a major challenge in diagnostics. The invasive procedures currently used are time-consuming, cumbersome and a major burden for patients. Early diagnosis is crucial for prevention and treatment. Particularly in the case of implants, rapid differentiation between septic and aseptic inflammation is essential and has a direct impact on treatment decisions. Non-invasive, specific imaging such as nuclear medicine positron emission tomography (PET) shows great potential here.
In this research project, new radiotracers are being developed that are specifically taken up by bacteria via a transporter. This transporter is not expressed on mammalian cells and therefore represents an exclusive target. The aim is to develop radiotracers for specific imaging of bacterial infections that allow differentiation from sterile tissue inflammation.