EX-VIVO ORGAN PERFUSION
PD Dr. Bettina Wiegmann
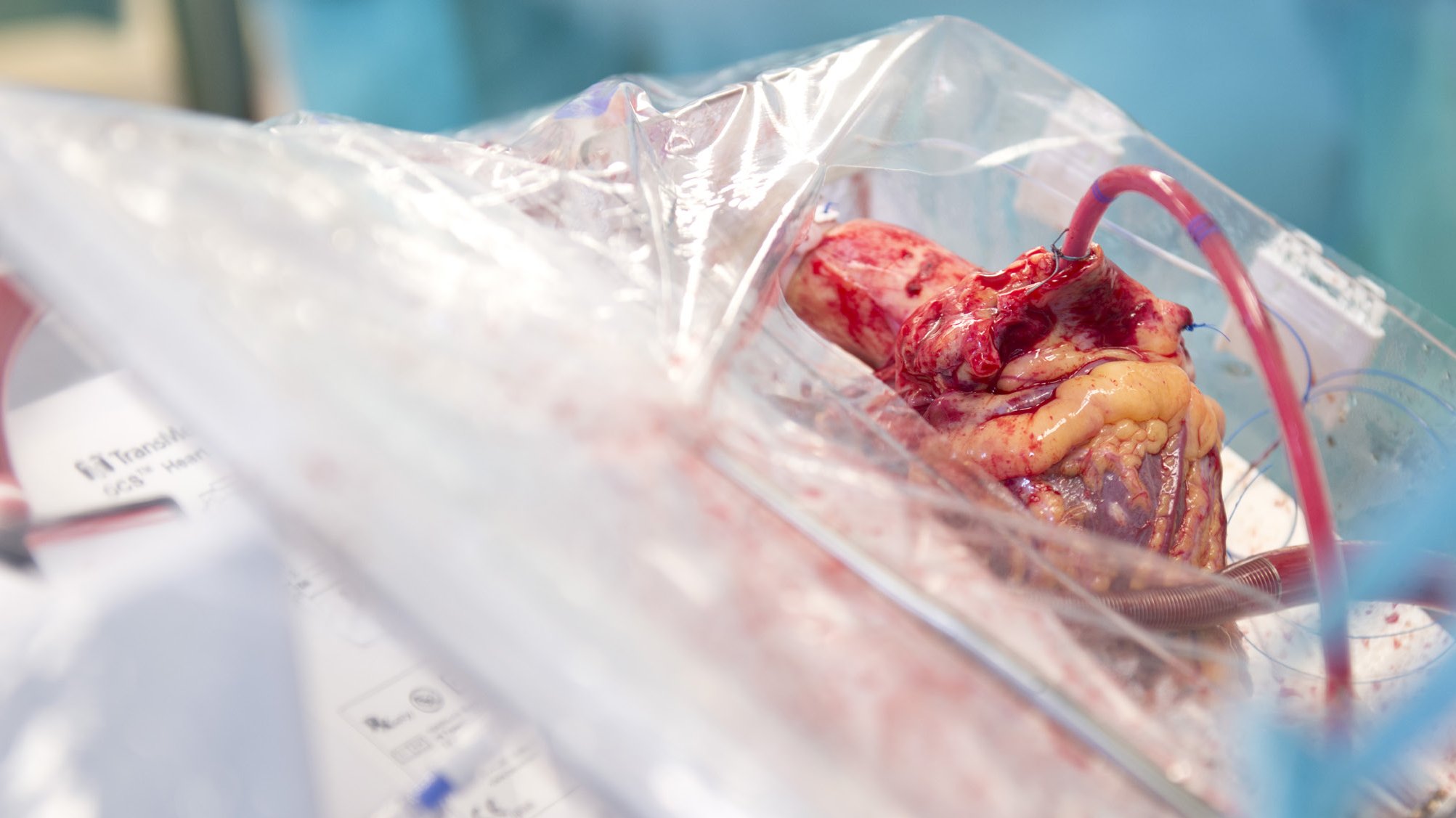
Due to the increasing discrepancy between organ recipients and organ donors, more and more patients on the waiting list for organ transplants are dying. In addition, ischemia-reperfusion injury during organ transplantation leads to acute and chronic rejection of transplanted organs. To address these problems, ex-vivo organ perfusion systems (e.g. heart, lung, liver, kidney) have been developed in recent years, which on the one hand allow greater geographical flexibility in donor management and enable the re-evaluation of potential donor organs, thereby increasing the number of available donor organs. On the other hand, the organs in the ex-vivo systems can be perfused (body) warm with nutrient solutions and thus contribute to the reduction of ischemia-reperfusion damage and consequently to an improvement of the results after organ transplantation. In addition to the optimization and expansion of the spectrum of ex vivo organ perfusion (e.g. limb perfusion) in the context of allogeneic (exogenous) transplantation, the further development of modern medicine has opened up a multitude of other possible interdisciplinary ex vivo therapeutic approaches, which the working group is working on developing and establishing. These are highly innovative strategies in the sense of regenerative, immunological, cell-based, pharmacological, anti-tumor and anti-infectious therapeutic approaches for the various organs and extremities that can be perfused ex-vivo in the context of autologous (autologous) replantations. Following the translational idea, the corresponding organ could be treated ex-vivo while the patient is connected to the corresponding organ replacement procedure (e.g. dialysis, extracorporeal membrane oxygenation) for this period for general stabilization. This is followed by autologous replantation of the organ previously treated ex vivo. In addition, the aforementioned ex-vivo therapy strategies are also established for use in allogeneic transplantations.
A) As part of interdisciplinary projects, the above-mentioned ex-vivo therapy approaches are compared and analyzed with those of in-vivo therapy - this is done in both large animal and small animal models
B) The extensive expertise of the working group enables the respective individual and project-specific adaptation of the ex-vivo perfusion devices, such as for the assessment of ex-vivo perfused organs (e.g. heart, lung) in CT and CT scans. heart, lungs) in CT and MRI
C) The ex-vivo application also enables the organ- and project-specific adaptation of the ex-vivo environment, e.g. the perfusion pressure, perfusion speed, perfusion solution (e.g. concentration of pO2, pCO2, haemoglobin) and the temperature can be varied accordingly
D) In addition, the working group focuses on the development, analysis and establishment of the following innovative therapeutic strategies, both in the field of allogeneic transplantation and autologous replantation:
a. Regenerative ex-vivo medicine/tissue engineering/cell-based therapy strategies
b. Ex-vivo tumor therapy (ex-vivo radiotherapy and high-dose chemotherapy)
c. Ex-vivo immunotherapy (e.g. ex-vivo MHC silencing)
d. Ex-vivo infection therapy (e.g. application of high-dose antibiotic therapy)
e. Ex-vivo gene therapy (e.g. ex-vivo correction of genetic diseases)
E) Advantages of ex-vivo compared to in-vivo therapy include the local, organ-specific limited therapy option, the absence of dose-limiting systemic side effects with the possibility of increasing the dose and a consecutive more effective therapy, as well as the project-specific adaptation of the ex-vivo environment (e.g. reduction of the pO2 content)
1.
Calabrese F, Schiavon M, Perissinotto E, Lunardi F, Marulli G, Di Gregorio G, Pezzuto F, Vuljan S, Forin E, Wiegmann
B, Jonigk D, MD, Warnecke G, Rea F; Organ Care System Lung resulted In Lower Apoptosis and INOS Expression
in Donor Lungs; Am J Transplant. 2020 Jul 11. doi: 10.1111/ajt.16187.
2.
Blockus S, Wiechert S, Wetzke M, Grethe C, Frenz T, Pils M, Prochnow H, Rox K, Wiegmann B, Dijkman
R, Rameix-Welti MA, Eléouët JF, Duprex P, Thiel V, Hansen G, Stadler M, Brönstrup M, Haid S, and Pietschmann
T; Labyrinthopeptins as virolytic inhibitors of respiratory syncytial virus cell entry; Antiviral Res. 2020 May;177:104774.
doi: 10.1016/j.antiviral.2020.104774. Epub 2020 Mar 18. PMID: 32197980
3.
Warnecke G, Van Raemdonck D, Smith MA, Massard G, Kukreja J, Rea F, Loor G, De Robertis F, Nagendran J,
Dhital KK, Moradiellos Díez FJ, Knosalla C, Bermudez CA, Tsui S, McCurry K, Wang IW, Deuse T, Lesèche G, Thomas
P, Tudorache I, Kühn C, Avsar M, Wiegmann B, Sommer W, Neyrinck A, Schiavon M, Calabrese F, Santelmo N,
Olland A, Falcoz PE, Simon AR, Varela A, Madsen JC, Hertz M, Haverich A, Ardehali A. Normothermic ex-vivo
preservation with the portable Organ Care System Lung device for bilateral lung transplantation (INSPIRE):
a randomized, open-label, non-inferiority, phase 3 study. Lancet Respir Med. 2018 May;6(5):357-367. doi: 10.1016/
S2213-2600(18)30136-X. Epub 2018 Apr 9.
4.
Figueiredo C, Carvalho Oliveira M, Chen-Wacker C, Jansson K, Höffler K, Yuzefovych Y, Pogozhykh O, Jin Z,
Kühnel M, Jonigk D, Wiegmann B, Sommer W, Haverich A, Warnecke G, Blasczyk R. Immunoengineering of the
Vascular Endothelium to Silence MHC Expression During Normothermic Ex Vivo Lung Perfusion. Hum Gene
Ther. 2018 Nov 20. doi: 10.1089/hum.2018.117.
5.
Sander J, Schmidt S, Cirovic B, McGovern N, Papantonopoulou O, Hardt A, Aschenbrenner A, Kreer C, Quast T,
Xu A, Schmidleithner L, Theis H, Do T, Sumatoh H, Lauterbach M, Schulte-Schrepping J, Günther P, Xue J, Baßler
K, Ulas T, Klee K, Katzmarski N, Herresthal S, Krebs W, Martin B, Latz E, Händler K, Kraut M, Kolanus W, Beyer
M, Falk C, Wiegmann B, Burgdorf S, Melosh N, Newell E, Ginhoux F, Schlitzer A, Schultze J. Cellular differentiation
of human monocytes is regulated by time dependent IL4 signaling and NCOR2. Immunity. 2017 Dec 19;47(6):1051-
1066.e12. doi: 10.1016/j.immuni.2017.11.024.
6.
Pflaum M, Kühn-Kauffeldt M, Schmeckebier S, Dipresa D, Chauhan K, Wiegmann B, Haug RJ, Schein J, Haverich A,
Korossis S. Endothelialization and characterization of titanium dioxide-coated gas-exchange membranes for
application in the bioartificial lung. Acta Biomater. 2017 Mar 1;50:510-521. doi: 10.1016/j.actbio.2016.12.017.
Epub 2016 Dec 9.
7.
Sommer W, Ius F, Salman J, Avsar M, Tudorache I, Kühn C, Wiegmann B, Marsch G, Kaufeld T, Zinne N, Fuehner T,
Greer M, Gottlieb J, Boethig D, Haverich A, Welte T, Warnecke G. Survival and spirometry outcomes after lung
transplantation from donors aged 70 years and older. J Heart Lung Transplant. 2015 Oct;34(10):1325-33. doi:
10.1016/j.healun.2015.06.002. Epub 2015 Jun 10.
8.
Wiegmann B, Figueiredo C, Gras C, Pflaum M, Schmeckebier S, Korossis S, Haverich A, Blasczyk R. Prevention
of rejection of allogeneic endothelial cells in a biohybrid lung by silencing HLA-class I expression. Biomaterials.
2014 Sep;35(28):8123-33. doi: 10.1016/j.biomaterials.2014.06.007. Epub 2014 Jun 21.
9.
Birschmann I, Dittrich M, Eller T, Wiegmann B, Reininger AJ, Budde U, Strüber M. Ambient hemolysis and
activation of coagulation is different between HeartMate II and HeartWare left ventricular assist devices. J Heart
Lung Transplant. 2014 Jan;33(1):80-7. doi: 10.1016/j.healun.2013.11.010. Epub 2013 Dec 1.
10.
Warnecke G, Moradiellos J, Tudorache I, Kühn C, Avsar M, Wiegmann B, Sommer W, Ius F, Kunze C, Gottlieb
J, Varela A, Haverich A. Normothermic perfusion of donor lungs for preservation and assessment with the Organ
Care System Lung before bilateral transplantation: a pilot study of 12 patients. Lancet. 2012 Nov 24;380(9856):1851-
8. doi: 10.1016/S0140-6736(12)61344-0. Epub 2012 Oct 10.
AG Biohybrid Lung
Dr. Bettina Wiegmann
Phone: +49 (0) 511 - 532 1408
Mail: wiegmann.bettina@mh-hannover.de