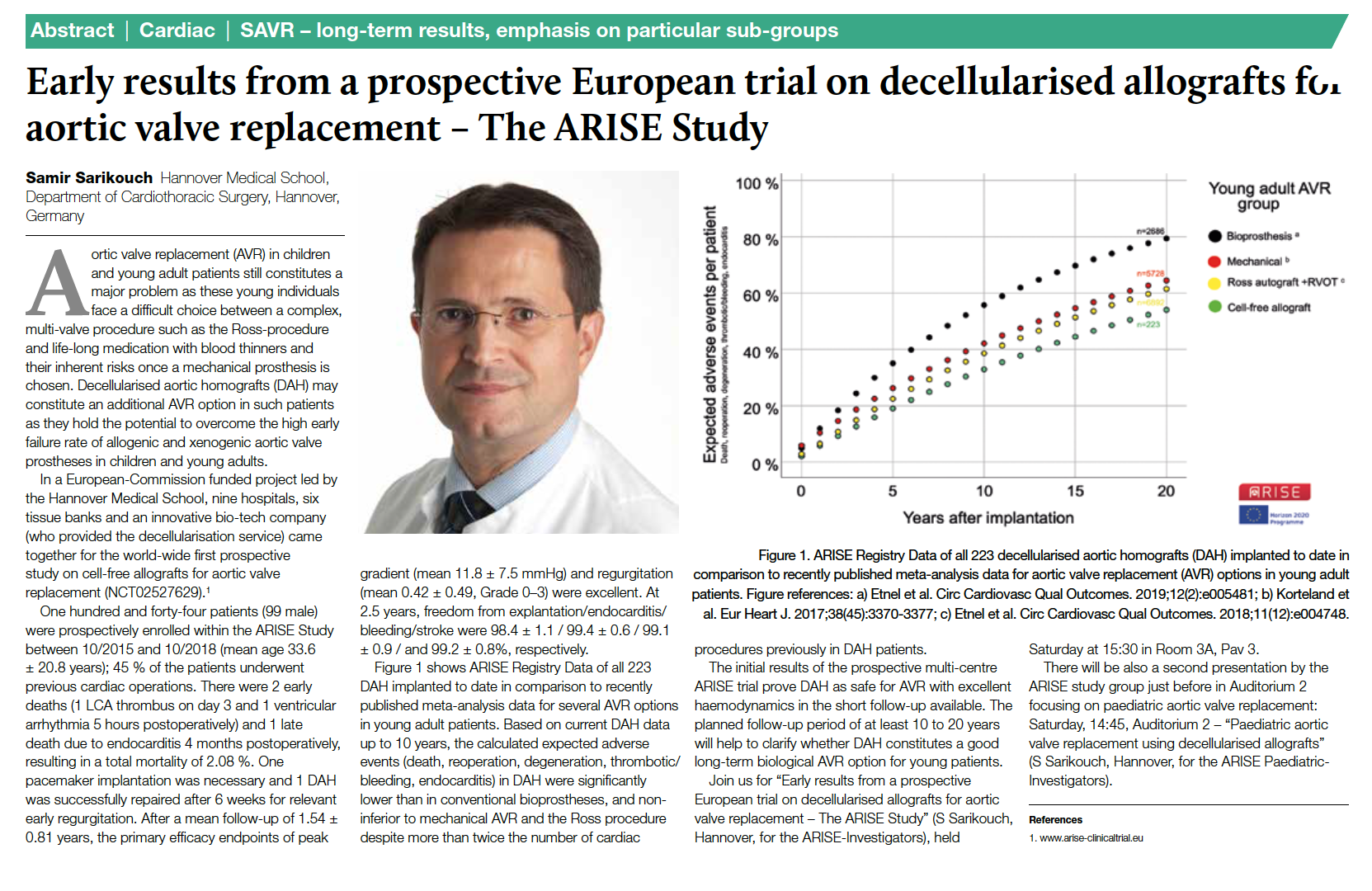
AORTIC CLAMP REPLACEMENT IN YOUNG PATIENTS
New treatment option successfully tested in Europe-wide study
Young patients in particular face a dilemma when choosing an aortic valve replacement procedure. Conventional biological, industrially produced heart valves made from animal tissue have a significantly shorter durability in younger patients (< 50 years), in contrast to the very good results in older patients. Mechanical heart valves require long-term treatment with blood thinners. The dangers associated with this medication, such as bleeding complications, add up to a considerable risk over the course of a lifetime, especially in younger patients. The so-called Ross operation as a third treatment option requires a two-valve procedure on the aortic and
pulmonary artery valve and is not feasible for all patients.
Cell-free human donor heart valves, so-called decellularized homografts, have been developed at Hannover Medical School and have now been successfully tested in a Europe-wide study funded by the European Commission¹. Cell-free heart valves represent an excellent new treatment option, particularly for patients with several previous operations or for patients with impaired left ventricular function. The results were presented in fall 2019 at the annual meeting of the European Association of Cardiac Surgeons (EACTS) and published in the corresponding journal.²
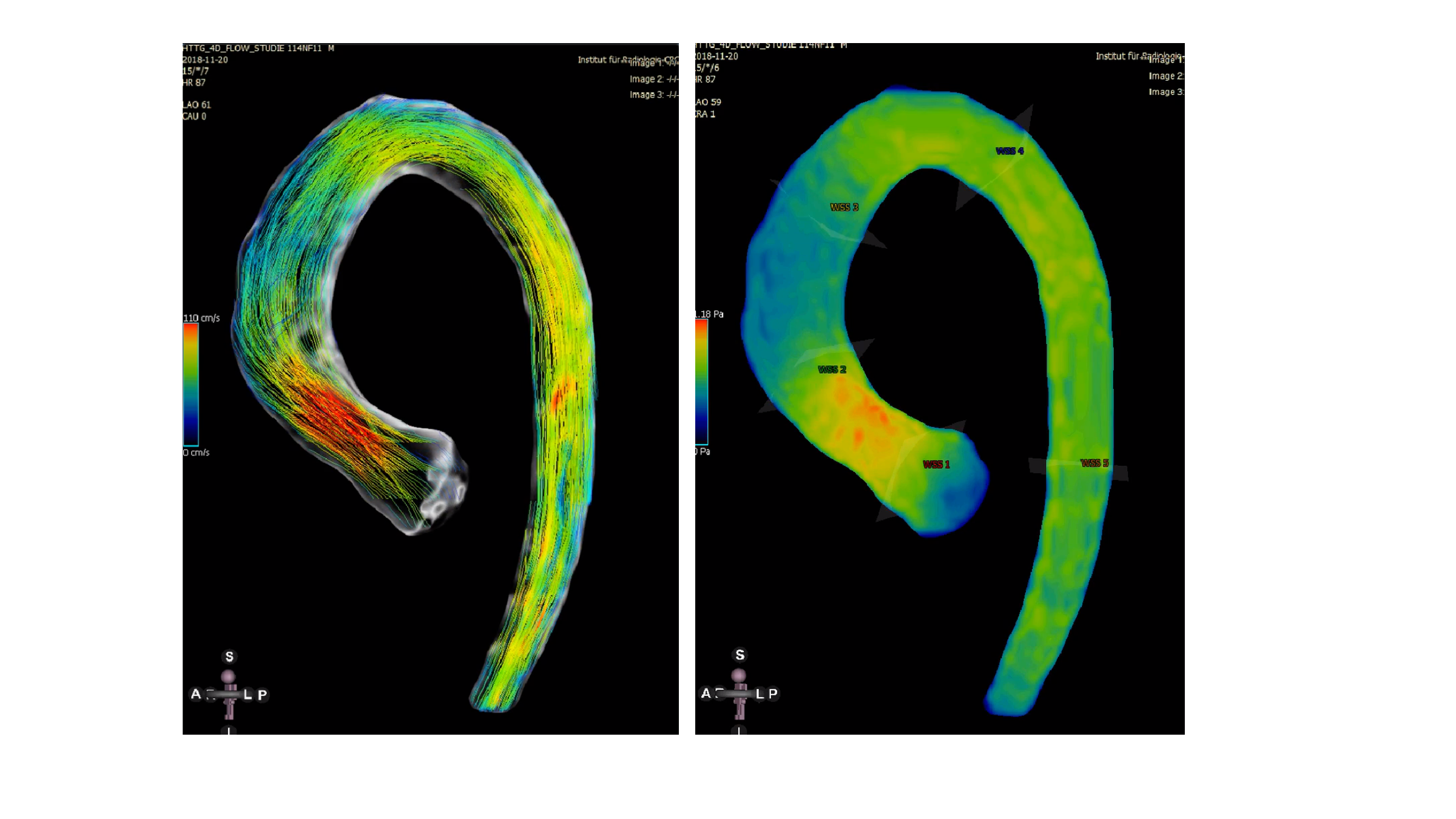
Fig. 1 shows the corresponding article from the EACTS congress journal from Lisbon. Fig. 2 shows 4D flow measurements after implantation of a long cell-free aortic valve replacement including the ascending part of the aorta. The flow lines (left) and the representation of the wall tension (right) are almost identical to those of healthy controls who did not undergo surgery.
The Clinical Department of Cardiac, Thoracic, Transplantation and Vascular Surgery (HTTG) under the direction of Prof. Dr. Axel Haverich has been dedicated to the clinical testing of new therapeutic strategies for the aortic valve and the aortic arch for many years. For example, the Clinical Department is collecting the results of one of the world's largest patient cohorts for the preservation of the body's own aortic valve, the so-called David reconstruction.
However, such new developments or further developments of existing medical products require careful examination before they can be used in the routine care of patients. HTTG dedicates a specially created area to this patient-oriented research.
The recording of long-term results is a focus of this clinical research. For example, in the above-mentioned study on cell-free aortic valves, a follow-up of at least 10 years is planned. This long-term observation represents the continuation of the protection of the study participants, which begins with detailed information about the planned study measure and intensive support during the study.
Cooperation with the approval and supervisory authorities forms a central part of the Clinical Research department at HTTG, which is supported by the central research infrastructure of Hannover Medical School, such as the Hannover Clinical Trial Center (HCTC) and the Quality Management in Clinical Research Administrative Unit. Regular internal and external training courses for medical and non-medical staff ensure compliance with national and European regulations and the quality of study results.
¹ www.arise-clinicaltrial.eu
² Horke A, Haverich A et al. Early results from a prospective, single-arm
European trial on decellularized allografts for aortic valve replacement - The
ARISE Study and ARISE Registry Data. Eur J Cardiothorac Surg. 2020, in press.
³ Beckmann E, Martens A, Krueger H, Korte W, Kaufeld T, Haverich A, Shrestha
ML. Aortic Valve-Sparing Root Replacement (David I Procedure) in Adolescents:
Long-Term Outcome. Thorac Cardiovasc Surg. 2019 Jul 22.