Laboratory for cerebrospinal fluid diagnostics and neurochemistry
Laboratory director:
Prof. Dr. med. Thomas Skripuletz
Deputy head of laboratory:
PD Dr. med. Philipp Schwenkenbecher
Technical staff:
I. Cierpka-Leja
K. Dorsch
K. Fricke
K. Scheiwe
The laboratory can be contacted by telephone on 0511 / 532-4056 or -3645
by FAX 0511 / 532-3577
You can print out the request form for our laboratory services as a PDF here
The analysis of cerebrospinal fluid is an important standard diagnostic procedure in neurological medicine. It is necessary for the diagnosis of acute inflammatory diseases (e.g. meningitis, encephalitis, myelitis) as well as chronic inflammatory diseases of the central nervous system such as multiple sclerosis or neuroborreliosis. In addition, the examination of cerebrospinal fluid can be used to detect bleeding and tumor cells. In recent years, the examination of cerebrospinal fluid has become even more important in the diagnosis of degenerative diseases such as dementia and motor neuron diseases.
The processing of cerebrospinal fluid in our own laboratory offers a high level of quality and, as a result, optimum results. The cerebrospinal fluid is processed within a short time. The evaluation of the cerebrospinal fluid requires special expertise and is only carried out by experienced employees who have been involved in the analyses for years.
Cerebrospinal fluid diagnostics
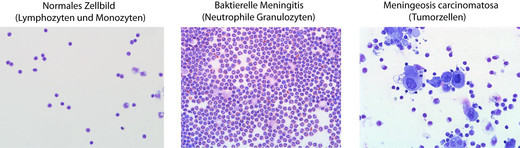
Cytology
Cell counting
Cells and erythrocytes are counted under a light microscope in the Fuchs-Rosenthal counting chamber.
Cell differentiation
The cell preparations are prepared using a cytocentrifuge. This is followed by staining according to Pappenheim (combination of May-Grünwald and Giemsa staining).
If necessary, immunocytological staining against corresponding surface markers is performed to further characterize suspect cells (tumour cells, etc.).
The following cells are differentiated:
Cells of the normal cerebrospinal fluid
Lymphocytes
Monocytes
Cells of the pathological cerebrospinal fluid
Activated lymphocytes, plasma cells
Activated monocytes
Macrophages (erythrophages, erythrosiderophages, siderophages, bacteriophages, leukophages, lipophages)
Granulocytes (neutrophils, eosinophils, basophils)
Tumor cells
Remaining cells
Cells of the epithelium (ependyma, choroid plexus)
Cartilage cells
Bone marrow progenitor cells
In addition, hematoidin crystals, bacteria and fungi are indicated, if present.
Protein analysis
Total protein (CSF)
Albumin, IgG, IgA and IgM (CSF and serum)
Both albumin and the immunoglobulins G, A and M are determined in the same run using nephelometric measurement. The measurement in the same run is a prerequisite for a precise evaluation in the quotient diagrams according to Reiber.
Oligoclonal bands
The oligoclonal bands in CSF and serum are determined by isoelectrofocusing on macro-polyacrylamide gels with automated silver staining.
Isoelectrofocusing enables the proteins to be focused according to their isoelectric point, resulting in very sharp bands. The use of polyacrylamide gels enables a further increase in resolution due to low endosmosis.
Autoimmune antibody diagnostics
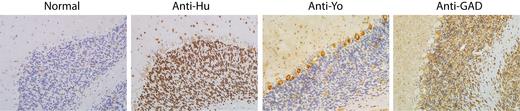
Antineuronal antibodies
Immunohistochemistry
This method uses cerebellar sections from monkeys to detect antineuronal antibodies. After applying serum or cerebrospinal fluid to the sections, immunohistochemical staining is performed (use of secondary antibodies against human IgG, peroxidase reaction). This method has the advantage over immunoblotting that it is less time-consuming. A further advantage is its use as a "screening", as only known antibodies can be examined in the immunoblot. Using immunohistochemistry, unknown antibodies or antibodies for which there is no detection possibility in the blot can be seen (e.g. the antibody Tr).
Immunoblot
Use of recombinant onconeuronal antigens.
The following antibodies are detected:
Hu
Yo
Ri
amphiphysin
CV2
Ma1
Ma2
GAD
Sox1
The immunoblot can be used primarily as a confirmatory test after the immunohistochemical method has been performed. This is necessary because cerebellar sections alone do not always allow an antibody to be clearly assigned. The immunohistochemical staining of tissue sections can simulate a staining pattern that resembles an antineuronal antibody due to a systemic antibody (e.g. ANA). However, the disadvantage of the blot is that only the known antibodies can be analyzed, unknown antibodies are not detected.
Autoimmune encephalitis antibodies (IgG)
Use of a BIOCHIP mosaic with transfected cells from EUROIMMUN. For the detection of certain autoimmune antibodies, transfected cells can be used that express the antigen against which the antibody to be tested binds and can be detected by immunofluorescence. Six different (separate) transfected cells are applied to the mosaic slide so that the antibodies against several antigens can be examined simultaneously:
Glutamate receptor ( NMDA type)
Glutamate receptor ( AMPA1 type)
Glutamate receptor ( AMPA2 type)
Contactin-associated protein 2(CASPR2)
Leucine-rich glioma-inactivated protein 1(LGI1)
GABA B receptor(GABAB)
Aquaporin-4 antibody (AQP4-IgG)
Myelin oligodendrocyte glycoprotein antibody (MOG-IgG)
The AQP4 and MOG antibodies of the IgG class are determined by indirect immunofluorescence on slides with transfected cells.
ELISA
The following antibodies are analyzed using the ELISA method:
GM1 ganglioside antibody (IgM)
MAG antibody (IgM)