Heart
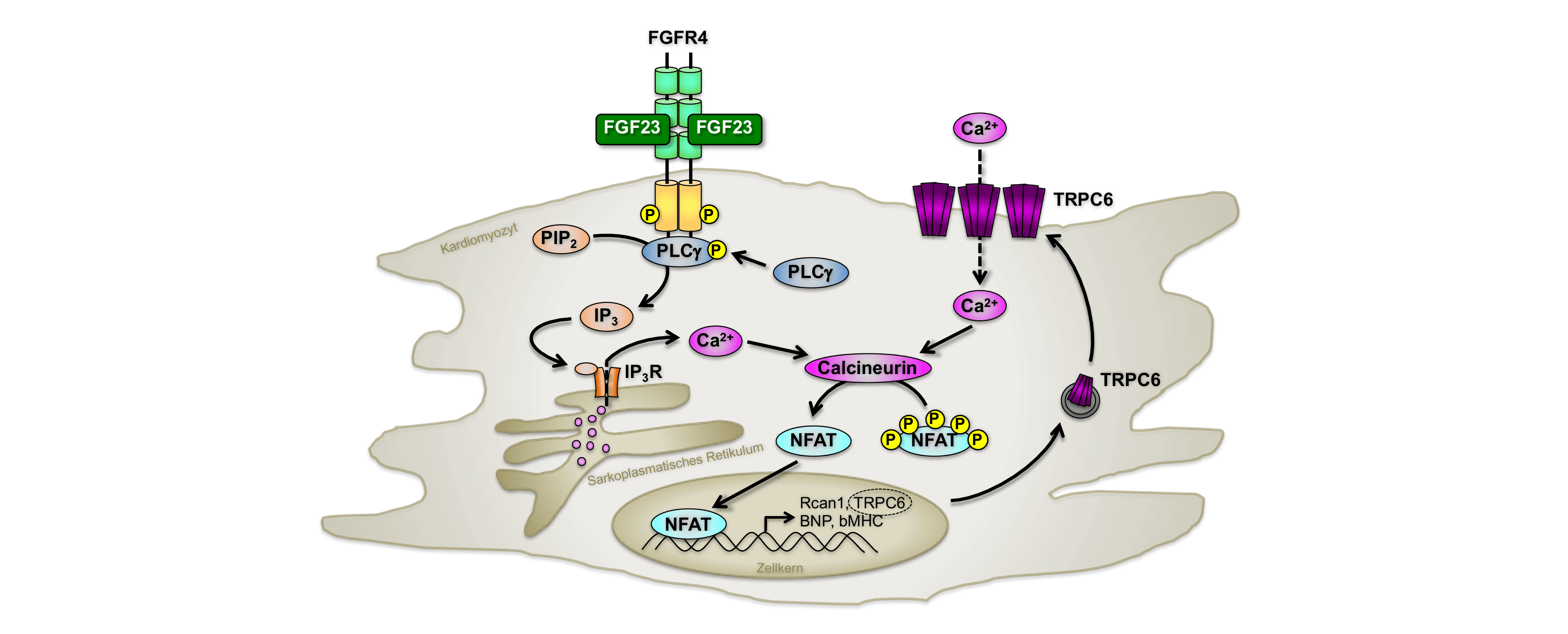
Elevated levels of fibroblast growth factor (FGF) 23 correlate significantly with the mortality rate and the occurrence of left ventricular hypertrophy (LVH) in patients with chronic renal failure, independent of known cardiovascular risk factors. In 2011, FGF23 was first shown to directly induce pathological hypertrophy via activation of the calcineurin/NFAT signaling cascade in cardiomyocytes and to induce LVH in mice (Figure). Shortly thereafter, FGFR4 was identified as a cardiac-specific receptor for FGF23-induced cardiac injury.
Regardless of the high systemic levels of FGF23, our research group was able to show an increase in intra-cardiac FGF23 synthesis in children with end-stage renal disease, which is significantly associated with the occurrence of LVH. In contrast to the well-described synthesis of FGF23 in bone, the importance of cardiac FGF23 in renal and cardiac disease and in the general population is controversial. To date, it is not clear whether cardiac FGF23 leads to a pathological cardiac phenotype or not in the absence of other underlying disease.
Our research group focuses on the age-dependent synthesis of FGF23 in cardiomyocytes, cardiac fibroblasts and endothelial cells, its physiological relevance and pathophysiological interaction. Using cardiac cell type-specific FGF23 knockout mice and the corresponding overexpression induced by cardiac gene transfer using adeno-associated viral vectors, we analyze to what extent cardiac FGF23 per se can trigger cardiac injury or whether other factors contribute to FGF23 cardiotoxicity. The focus here is on investigations into high blood pressure and impaired mineral and bone metabolism.
Sub-projects on this complex of topics are funded by a Klaus-Georg-and-Sigrid-Hengstberger research grant from the German Society of Cardiology (DGK).
Cardiovascular mortality is greatly increased in patients withchronic kidney disease (CKD). Left ventricular hypertrophy (LVH) is the most common cardiovascular disease in these patients and promotes diastolic dysfunction, congestive heart failure, cardiac arrhythmias and sudden death. Elevated levels of the phosphaturic hormone fibroblast growth factor (FGF) 23 are associated with the occurrence of LVH, endothelial dysfunction and increased cardiovascular mortality in CKD patients and in the general population. The most important stimuli for FGF23 are phosphate, calcitriol and parathyroid hormone (PTH).
In a secondary analysis of a randomized clinical trial with CKD patients, it was shown that PTH and FGF23 levels can be significantly reduced by treatment with calcimimetics. In addition, the reduction of FGF23 was significantly associated with a lower rate of cardiovascular deaths and cardiovascular events. Recently, a novel calcimimetic has become available for the treatment of secondary hyperparathyroidism that has been shown to effectively reduce circulating PTH and FGF23 levels by up to 70% in uremic rats and dialysis patients. The cardioprotective properties of this new calcimimetic have not yet been investigated and the exact mechanisms of cardioprotection are poorly understood.
In a mouse model of phosphate-induced cardiac injury, our group is investigating the dose-dependent effects of this calcimimetic on calcium-phosphate metabolism and circulating PTH and FGF23 concentrations. Functional and molecular biological analyses will provide information on the type of cardiac damage at the start of therapy and characterize the mechanisms of cardioprotection after therapeutic intervention with the calcimimetic.
This third-party funded project is supported by AMGEN.
The most common cause of death in patients withchronic kidney disease (CKD) is cardiovascular in origin, with left ventricular hypertrophy (LVH) being the most common cardiovascular disease. As CKD progresses, plasma levels of fibroblast growth factor 23 (FGF23) increase progressively, while there is a parallel reduction in its cofactor Klotho. In the kidney, FGF23 suppresses the synthesis of active vitamin D (1,25(OH)2D3, calcitriol) by inhibiting Cyp27 and increases its degradation via the induction of Cyp24. As CKD progresses, the secretion of FGF23 increases and calcitriol deficiency occurs.
So far, many therapeutic options have been discussed to improve FGF23-associated complications in CKD patients. The positive impact of active vitamin D on cardiovascular pathophysiology in adult CKD patients is controversial. Possible cardioprotective properties of calcitriol in children with CKD have not yet been sufficiently investigated. There are initial indications that renal klotho expression can be increased by calcitriol or ACE inhibitors. Since both factors, calcitriol and Klotho, are thought to have a cardioprotective effect, our research group is investigating the influence of active vitamin D on the development of LVH and the FGF23/Klotho system. The findings from clinical studies are verified in vivo using the rat model of experimental uremia and the direct influence of calcitriol and Klotho on FGF23-induced hypertrophy is investigated in vitro at the molecular level.
Individual subprojects of this complex of topics are funded by the European Society for Paediatric Nephrology (ESPN) and supported by an internal university performance funding (HiLF) of the MHH.
Activation of the renin-angiotensin system (RAS) favors the development of left ventricular hypertrophy (LVH). Among other things, angiotensin II (AngII) leads to peripheral vasoconstriction, activation of the sympathetic nervous system and, via the angiotensin II receptor type 1, to the secretion of aldosterone from the adrenal glands. Aldosterone increases sodium and H2O reabsorption in the distal renal tubules, which increases blood pressure.
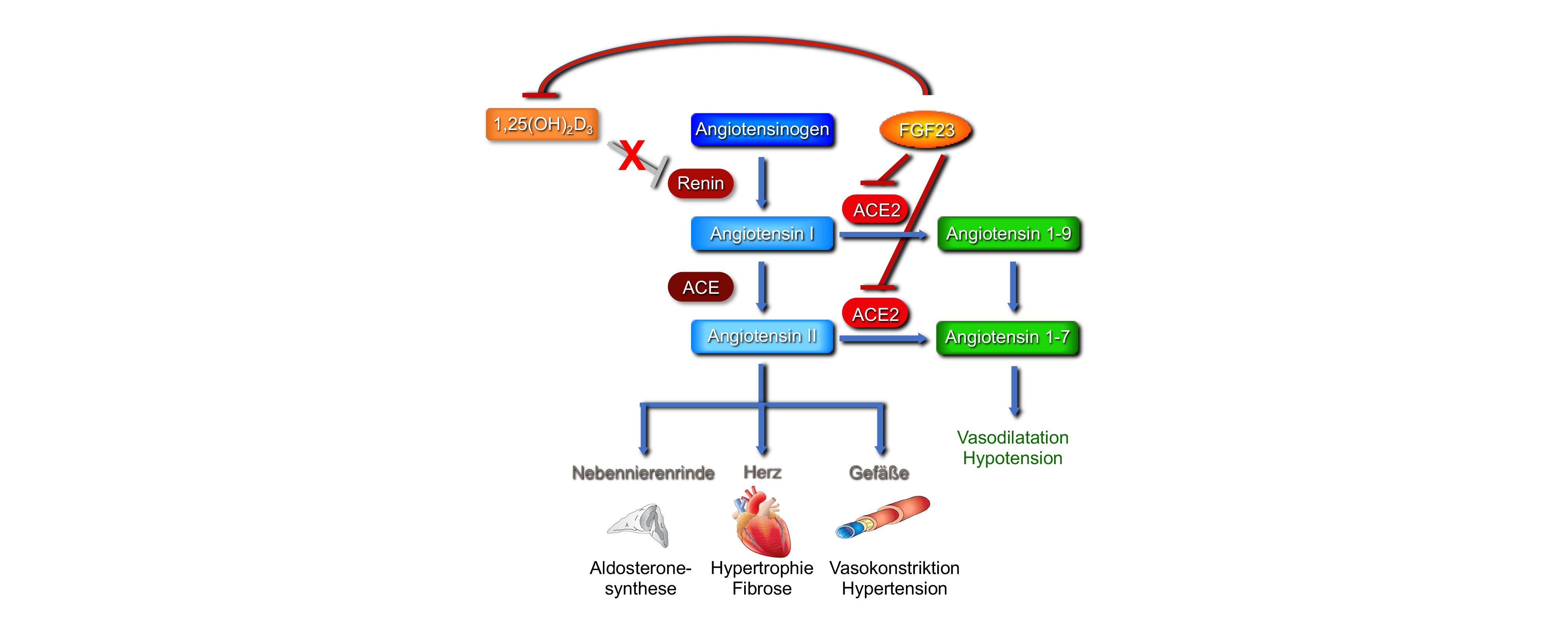
Apart from the well-characterized systemic RAS, various tissues, including the heart, show local RAS activation. Recently, fibroblast growth factor (FGF) 23 has also been linked to RAS activation. AngII and aldosterone increase circulating FGF23 plasma levels in rodents and AngII induces intracardiac FGF23 synthesis. Recent studies suggest that FGF23 activates the RAS by inhibiting ACE2, an enzyme that converts AngII to the vasodilatory and cardioprotective Ang 1-7. Indirectly, FGF23 may also contribute to the stimulation of renin, which is physiologically inhibited by active vitamin D, by inhibiting renal 1,25(OH)2D3 synthesis (Figure). This project aims to confirm the underlying molecular mechanisms and to elucidate the role of intracardiac RAS in FGF23-induced cardiac injury in vitro and in vivo.
Kidney
In patients withchronic kidney disease (CKD), a decrease in the glomerular filtration rate (GFR) leads to an altered mineral metabolism. Even with a slight reduction in GFR, serum phosphate levels rise progressively, resulting in increased secretion of the phosphaturic hormone fibroblast growth factor (FGF) 23 from the bone and, in later stages, increased synthesis of parathyroid hormone. Klotho is the physiological cofactor of FGF23 and triggers the activation of FGF23/Klotho/FGF receptor-mediated phosphorylation of MAP kinase ERK1/2 in the kidney, leading to inhibition of renal sodium-phosphate cotransporters and ultimately to reduced phosphate reabsorption in the renal tubules. As CKD progresses, the system decompensates, renal clot synthesis decreases and the binding affinity of FGF23 to its receptor is reduced, resulting in renal FGF23 resistance.
To date, the exact mechanism of phosphate sensing is unknown and, irrespective of an underlying renal disease, a high phosphate load is also associated with multiple organ dysfunctions, including kidney dysfunction, in the general population. The central focus of this project is the analysis of renal phosphate handling in chronic phosphate load. The interaction of the individual phosphate transporters (NaPi2a, NaPi2c, PiT-1, PiT-2) as well as the FGF23-dependent activation of specific signal transduction pathways in the kidney will be elucidated and contribute to a better understanding of the maintenance of phosphate homeostasis in the healthy organism.
An increased intake of inorganic phosphates (Pi) is a significant health risk for the general population due to the increased consumption of industrially processed foods. While epidemiologic studies show a clear association between a phosphate-rich diet and cardiovascular and all-cause mortality, the underlying pathomechanisms are unknown. New data from our group show that a high-phosphate diet in healthy mice increases phosphate excretion, stimulates plasma levels of the phosphaturic hormone fibroblast growth factor (FGF) 23 and leads to proximal tubule damage associated with increased infiltration of inflammatory cells (Figure).
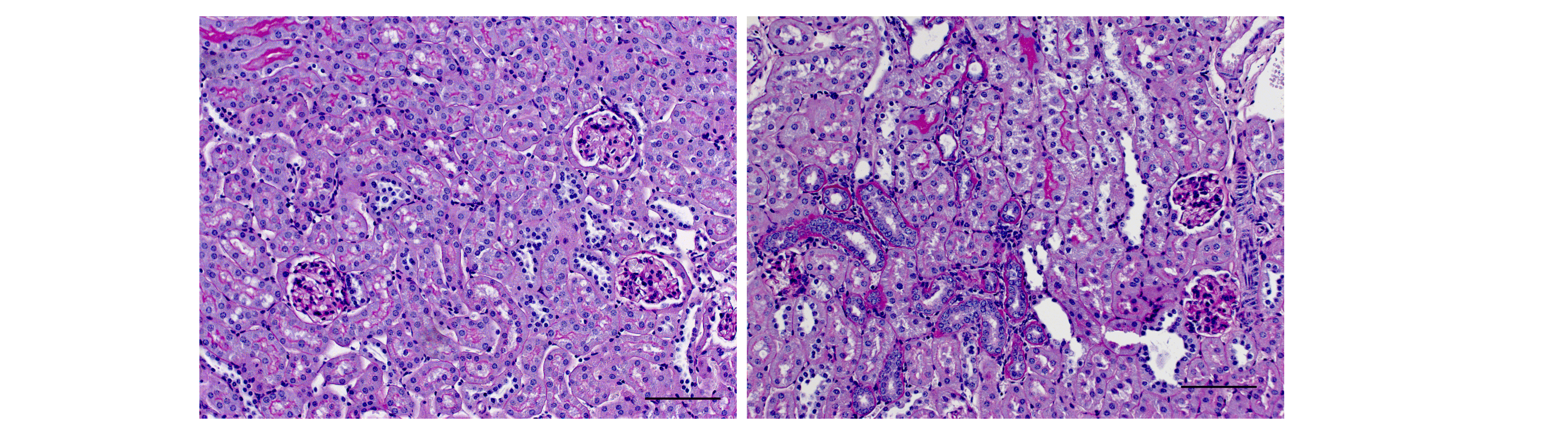
The focus of this project is the characterization of the nephrotoxicity of Pi in vivo and the identification of the underlying molecular mechanisms in proximal tubule cells in vitro. These investigations should sensitize for prevention and open up possible perspectives for therapeutic intervention.
The investigations on this complex of topics are supported by an internal university performance funding II (HiLF II) of the MHH.
Tertiary lymphoid organs (TLOs) are inducible structures that occur in peripheral, non-lymphoid organs and have an organization similar to that of secondary lymphoid organs. TLOs consist of parts of immunological cells, lymphatic and blood vessels, as well as tissue-bound fibroblasts and are associated with antigens and inflammatory processes. In the kidney, TLOs have been detected after transplantation, inchronic kidney disease (CKD) and in ageing kidneys. Initial data from our working group show an increased formation of renal TLOs due to a chronically high phosphate load (Figure), as is regularly observed in CKD patients, and suggests that phosphate favors an inflammatory milieu and also the progression of TLOs.
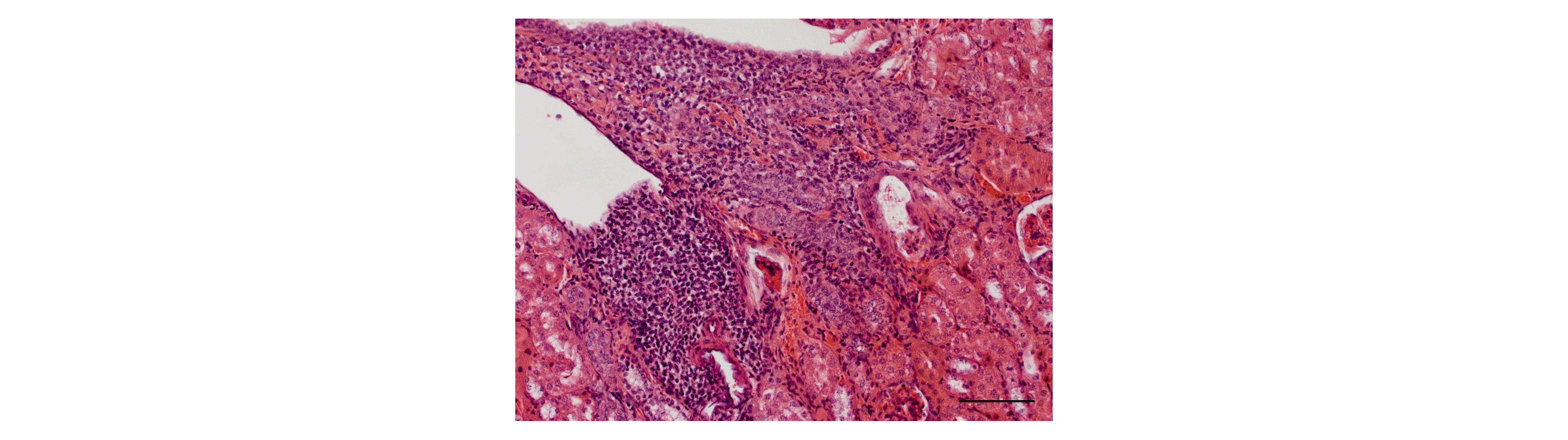
In this nephrological-immunological project, the molecular basis of phosphate-induced TLO development is to be worked out, and chemokines and other markers for the progression of TLOs are to be identified. The definition of typical biomarkers of TLO maturation should enable targeted therapy development in the long term.