Hannover Heart Rhythm Center at MHH implants Germany's first innovative defibrillator with an electrode under the sternum.
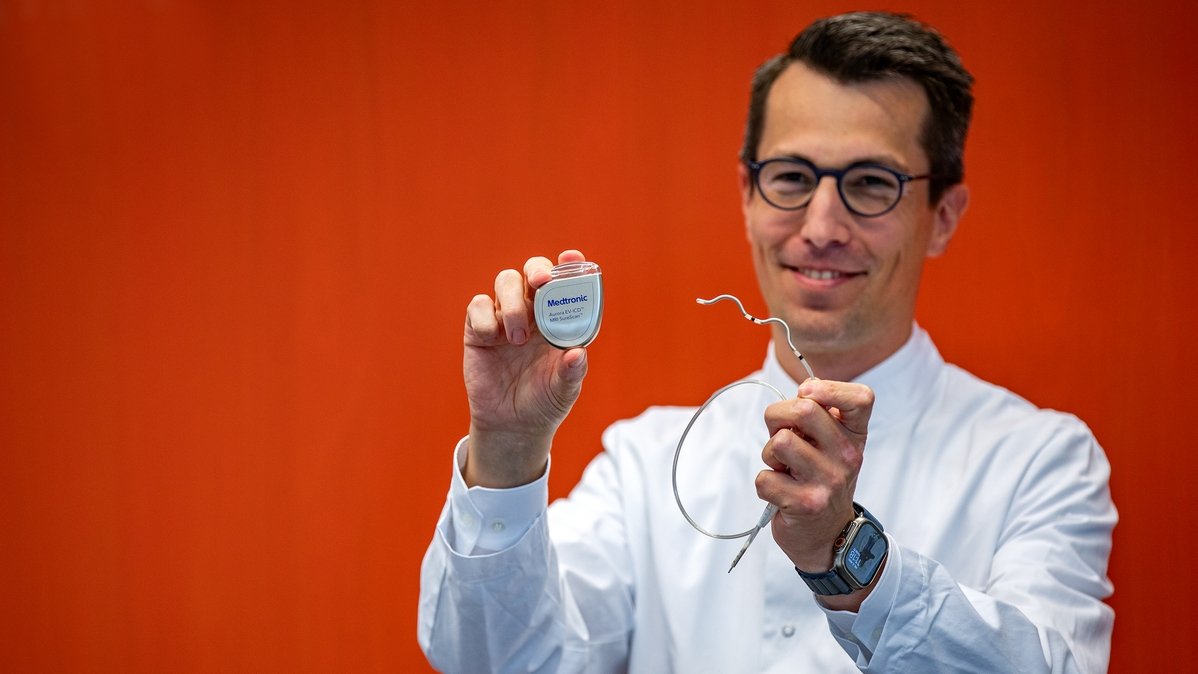
Professor Duncker shows the "Aurora" defibrillator and the corresponding electrode. Copyright: Karin Kaiser/MHH
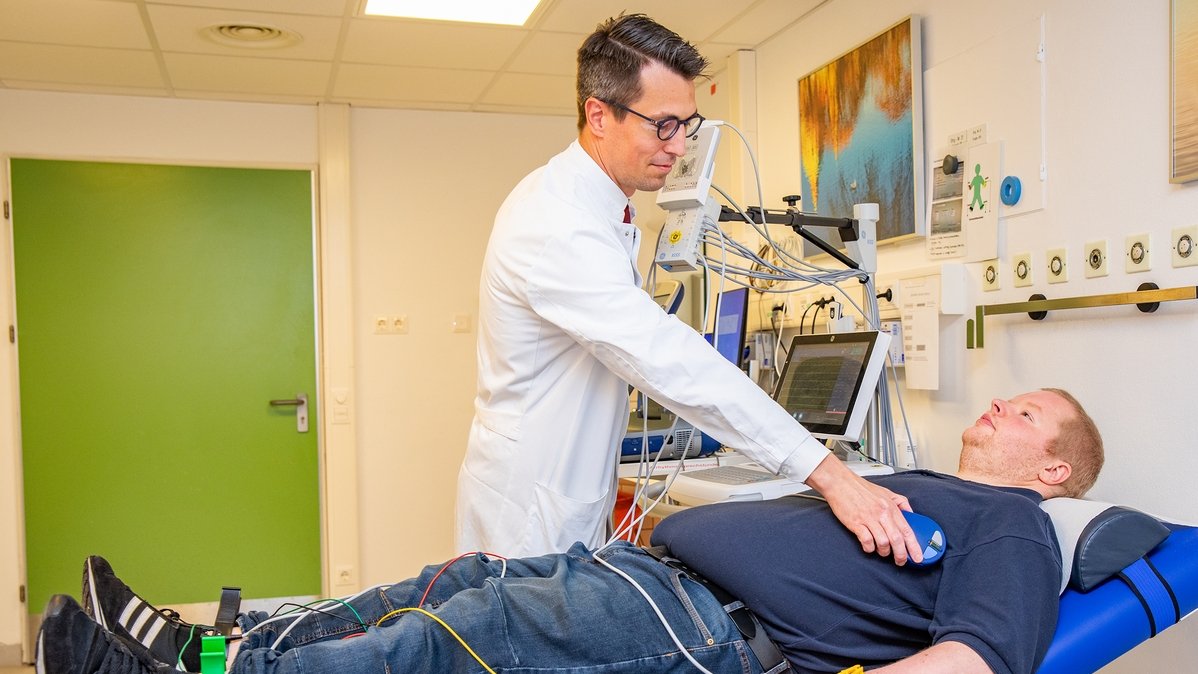
Follow-up examination: Professor Duncker checks whether the implanted device is working. Copyright: Karin Kaiser / MHH
In Germany, around 65,000 people die of sudden cardiac death every year. A common cause is ventricular fibrillation. This life-threatening cardiac arrhythmia can be stopped by a defibrillator. Hannover Medical School (MHH) is one of the pioneers in the implantation of defibrillators. Almost 40 years ago, the MHH was the first clinic in Germany to successfully implant a defibrillator immediately and completely. The technology has continued to develop over the years. Now a team from the Hannover Heart Rhythm Center of the MHH Cardiology Department has implanted a new type of system for the first time in Germany. In the extravascular implantable cardioverter defibrillator (EV-ICD) called Aurora, the electrode is located under the sternum. This creates advantages for certain patients.
Conventional models have weak points
Conventional defibrillators (ICDs) are implanted transvenously, i.e. a wire-shaped electrode is guided from the device below the collarbone via a larger vein into the heart, where it is firmly placed. If a malignant cardiac arrhythmia occurs, the defibrillator emits either several weak stimulation pulses or an electric shock and restores the heartbeat to the correct rhythm. This prevents sudden cardiac death. "The system is well established, the indications are clear and the technology is well developed," says Professor Dr. David Duncker, Head of the Hannover Heart Rhythm Center at the Clinic for Cardiology and Angiology. Nevertheless, there are known weaknesses. "For example, the lead can break over time, lead to infections or cause injury to the lungs during implantation," explains the cardiologist. To eliminate these disadvantages, so-called subcutaneous ICDs were developed a few years ago, in which the electrode is placed under the skin on the sternum. These ICDs can also defibrillate. However, as the electrode has no direct contact with the heart, cardiac arrhythmias are detected differently. The devices also lack a pacemaker function, which may be necessary if the heartbeat is too slow.
Successful further development
The new system now appears to combine the advantages of the transvenous and subcutaneous defibrillator and eliminate some of the disadvantages of both. The Aurora EV-ICDTM system from Medtronic is implanted extravascularly, i.e. outside the heart and veins. The cardiologists place the electrode under the sternum. In this way, the electrode lies on the heart. Direct contact enables the device to better detect cardiac arrhythmias and react accordingly. If the heartbeat is too fast or irregular, it first sends out small electrical signals in quick succession to correct the heart rate. Only if the disturbances persist does the device emit an electric shock. The experts call these small, rapid signals antitachycardic pacing (ATP). "This preliminary stage means that many shocks, including painful ones, can be avoided. This is a great advantage," emphasizes Professor Duncker. In addition, the Aurora EV-ICD system has a pacemaker function to stimulate the heart if the heartbeat is too slow or paused. "The new system is a successful further development of subcutaneous ICD systems and a good alternative for patients for whom a transvenous system is not well suited," explains Professor Dr. Johann Bauersachs, Director of the Department of Cardiology and Angiology. He is thinking in particular of people with a high risk of surgery, susceptibility to infection and leaky heart valves.
Premiere in Hanover
On October 13, 2023, Professor Duncker implanted the first Aurora defibrillator in Germany in a patient. One week later, he performed the second implantation of this type. The second patient is Bastian K. from Hanover. The 37-year-old suffers from heart failure. In May of this year, his heart's pumping capacity was only 10 percent. Every movement exhausted him. "After walking 100 meters, I was completely exhausted," he recalls. To protect himself from sudden cardiac death, Bastian K. had a defibrillator vest, which is worn directly on the skin under clothing and delivers an electric shock if necessary. "When the topic of implantation became concrete, I was presented with three possible devices with all the advantages and disadvantages. Everything was explained to me really well," says the patient. The decision was made in favor of the new Aurora defibrillator system. This device now gives Bastian K. a feeling of security.
Bastian K. was discharged from hospital one day after the implantation. In addition to the medical and nursing specialists from the cardiology and anesthesiology departments, the surgical team also included a cardiac surgeon. Privatdozentin (PD) Dr. Jasmin Hanke from the Clinic for Cardiac, Thoracic, Transplantation and Vascular Surgery was present as a backup during the first implantations of the new defibrillator. "We support each other as a team with our respective core competencies in cardiology and surgery. This enables us to offer our patients the maximum possible safety," explains PD Dr. Hanke. The cardiac surgeon also considers the Aurora device to be a good alternative to conventional defibrillators for certain patients. "In the long term, fewer complications can be expected with the devices."
Progress in patient care
Around 150 defibrillators are implanted in the Department of Cardiology and Angiology every year. Various models are used, depending on the type of cardiac arrhythmia and the needs of the patient. "We attach great importance to high-quality patient care and want to offer the latest clinical advances in the treatment of heart conditions. The new technology is a further step forward in this respect," says Professor Dr. Johann Bauersachs.
Worldwide approval study
The safety and efficacy of the Aurora EV-ICDTM System was evaluated in a global pivotal trial in patients at risk of sudden cardiac death. It involved 356 patients in 46 hospitals in 17 countries in North America, Europe, the Middle East, Asia, Australia and New Zealand. In August of this year, the system received approval in Europe.
Text: Tina Götting