Our research laboratories
Basic oncological research
"Translational medicine" or "molecular medicine" are the terms that best describe the activities in the field of uro-oncology research. The focus here is on the desire to be able to integrate molecular information into clinical-oncological decision-making processes in the future. The improvement of tumor detection (diagnosis), an optimized prediction of the individual course of the disease (prognosis) and finally the most precise assessment of the response to therapy are the goals of our research efforts.
The basis for this is the enormous increase in molecular information from various genome-wide analyses, i.e. analyses covering the entire human genome. These now allow a more refined description of the molecular changes in human tumors and especially in prostate, urinary bladder and renal cell carcinomas, which are important for urology.
The knowledge of changes in gene sequences that lead to the production of defective proteins represents an important area in molecular oncology. Recently, however, an increasing number of changes have been found that affect the correct regulation of the production of intrinsically correct proteins. Despite intact gene sequences, this can lead to the production of an insufficient quantity of the respective protein and thus to a loss of function and, to put it simply, the development of tumor diseases is the consequence of such and other molecular deviations. Overall, these are likely to take on a characteristic form for specific tumours in terms of type, extent and temporal sequence and could therefore form the basis for future personalized uro-oncology.
The uro-oncology working group focuses on collecting information on the altered control of gene expression, referred to as "epigenetics" in technical jargon, in urological tumors and examining its translational benefits.
"Epigenetics" is a collective term that includes all those processes that enable body cells to react to external, e.g. environmental influences. Such a reaction of a cell can, for example, consist of a changed reading of the genetic material and conversion (gene expression) into protein products. Interestingly, a new transcription program can be passed on to daughter cells, i.e. inherited within a tissue, but can also be reversed if necessary. The transmission of the information of the reading and conversion program is also ensured by means of chemical marking of the DNA, which takes place by attaching small molecular groups, the so-called methyl residues. This methylation usually takes place at specific labeling sites and can then influence the expression of the affected gene.
A good example of the importance of epigenetic processes in biology can be seen in honey bees. Changing the diet of bee larvae to "royal jelly" is known to cause the growth of queen bees in place of the workers produced by a normal diet. In both cases, the initial organism is genetically similar, i.e. in terms of the genetic information that is written as a sequence in the DNA, but an organism with a strikingly different appearance and abilities, which it can also maintain until its death, can be produced. As we know today, epigenetic changes caused externally by specific nutrition are responsible for this.
What can epigenetics do for uro-oncology?
It was of great interest to tumor biology when a large number of studies in recent years were able to demonstrate that, in addition to genetic changes such as gene mutations, epigenetic changes are very often also detectable in tumor cells. Subsequent studies have then shown that prostate, renal cell and urinary bladder carcinomas also exhibit such changes with great frequency.
A central question for translational research is now whether epigenetic marks can be translated into useful information for patients (translational medicine) and could therefore contribute to improving diagnosis, prognosis or treatment response in the future.
Based on genome-wide database comparisons and measurements on tumor cells and tissues, uro-oncological research has been able to identify a number of epigenetic marks with potential significance for urological tumors. The first results of this current series of investigations have recently been published or are still in the publication process.
From comparisons of epigenetic measurements from tumor tissues and the clinical course of affected patients, for example, it can already be deduced that the detection of certain methylation changes could be a very promising way of assessing the prognosis of patients with renal cell carcinoma. However, before any clinical application is possible, it will be necessary to collect independent data in prospective studies and in collaboration with other medical centers.
Other current projects include the recording of several DNA methylation markers, which together represent so-called methylation profiles. These are to be recorded using cell material isolated from urine samples. The aim of these investigations is to check whether the creation of methylation profiles from non-invasive sample material could allow an improved diagnostic or prognostic assessment of bladder and prostate carcinomas.
In uro-oncological research, the question of whether epigenetic changes in the form of DNA methylation could measurably increase the risk of tumor disease is also being pursued.
Interestingly, our research group was recently able to measure an epigenetic tumor risk for solid tumors for the first time ever. We were able to show that methylation in a specific section of the SFRP1 gene is statistically associated with a significantly increased risk of developing renal cell carcinoma.
Apart from its importance for the fundamental tumor biological understanding of carcinogenesis, the identification and quantification of the epigenetic risk contribution could thus form the basis for future individual tumor risk prediction.
The question of whether certain gene variants - i.e. changes in the human DNA sequence - are linked to an increased risk of developing prostate cancer is often investigated in large-scale international study consortia.
The urological oncology working group has so far taken part in two large-scale studies involving the examination of over 50,000 people and has thus contributed to the identification of several previously unknown sequence loci with significance for the development of prostate cancer.
The modern analytical methods available in uro-oncological research make it possible to obtain information on changes in gene expression, DNA methylation and the detection of sequence changes in genetic material from various test materials.
Isolation of sample materials
Robot-based automated isolation of DNA from fresh tissue samples, archive materials and body fluids.
Automation of measurement procedures
Robot-assisted preparation of sequence amplification reactions (polymerase chain reaction, PCR) in a high-throughput process.
Measurement of gene expression
PCR, western blotting and double immunofluorescence methods
Methylation analyses
DNA methylation analyses using pyrosequencing, quantitative real-time PCRs in high-throughput format or ultra-deep bisulfite sequencing with next-generation high-throughput sequencers.
Functional analyses
Real-time detection of gene-specific mediated functional changes by measuring the growth, mobility and invasion behavior of urological tumor cells through specific manipulation of gene expression.
Physiological-pharmacological basic research
We conduct basic research with the aim of characterizing signal transduction pathways and cellular receptors that control the normal function of the organs of the lower urinary tract (urinary bladder, prostate, urethra) and essential anatomical structures of the male and female reproductive system (penis, seminal vesicles, vagina, clitoris).
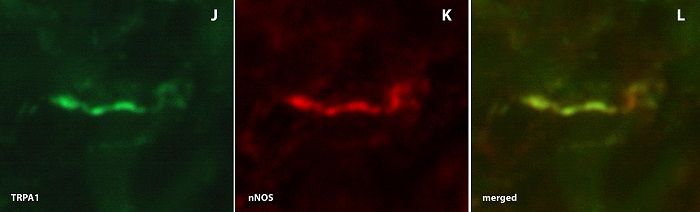
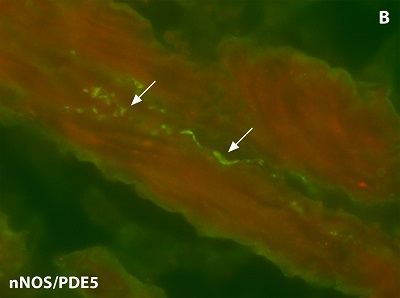
This work enables the identification of new strategies and active substances for the treatment of diseases of the lower urinary tract (overactive bladder, benign prostatic hyperplasia, lower urinary tract symptomatology) and sexual dysfunctions in men and women (erectile dysfunction, ejaculatio praecox, female sexual dysfunction). Possible targets of pharmacological influence are intracellular proteins (e.g. enzymes such as fatty acid amide hydrolase (FAAH) or the group of phosphodiesterases), membrane channels such as the transient receptor potential cationic channel A1 (TRPA1) and endogenous peptides (vasoactive intestinal polypeptide, C-type natriuretic peptide, calcitonin gene-related peptides).
The spectrum of methods used includes molecular biological protocols (Real Time Polymerase Chain Reaction), immunohistochemistry, radioimmunometric procedures (RIA) and experiments with isolated smooth muscle of the urinary bladder, prostate, urethra and seminal vesicles as well as the corpus cavernosum penis. The project work is being carried out in cooperation with the Institute of Biochemical Research & Analytics (IBFA, Barsinghausen), the Urological Research Institute of the Catholic University Vita Salute San Raffaele (Milan, Italy) and the Department of Clinical Pharmacology at Linköping University (Sweden).