Clinical studies
Investigation of new therapies and treatments
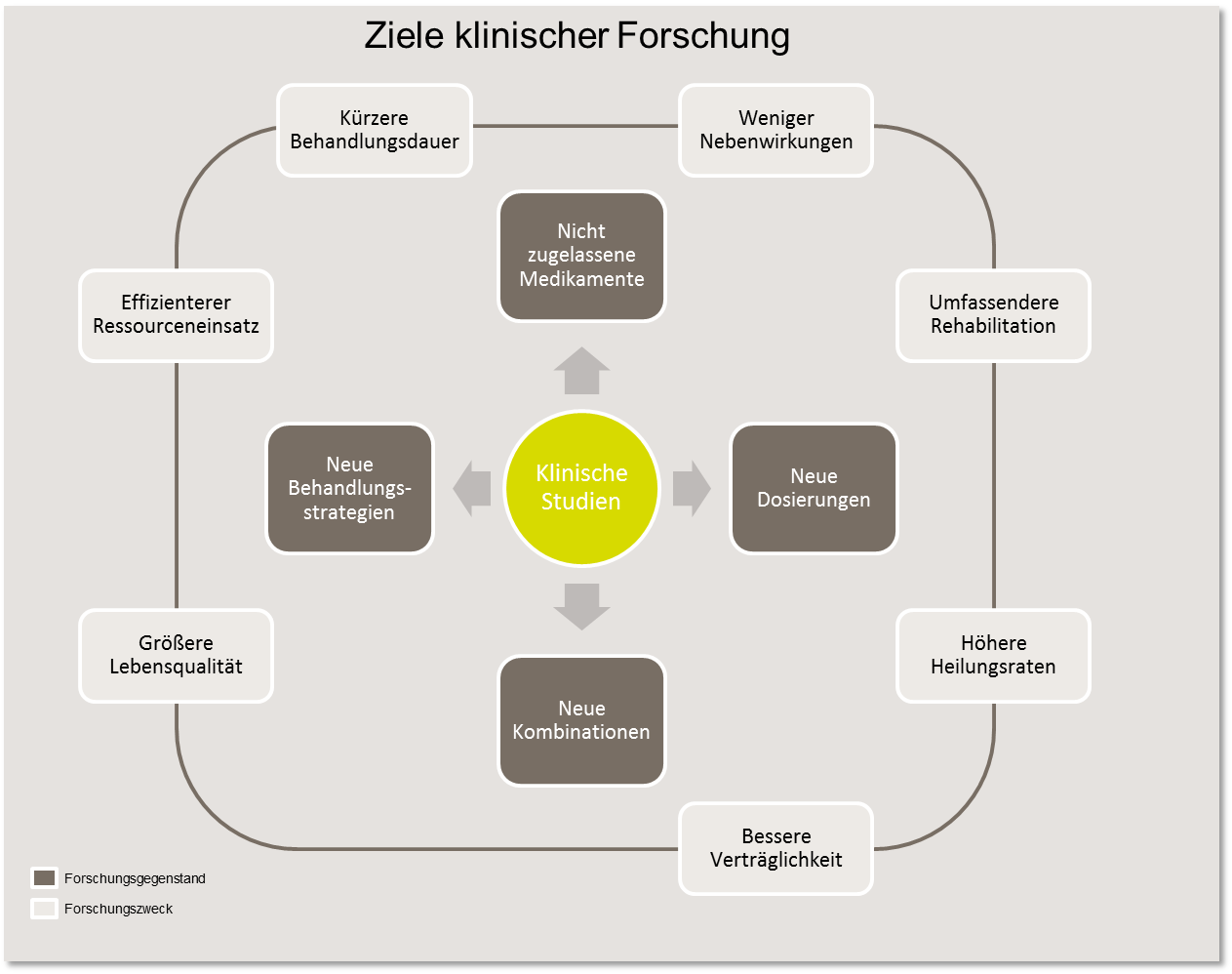
In clinical trials, new and not yet approved drugs are investigated, new combinations or dosages of already approved drugs are analyzed ornew treatment strategies for cancer (e.g. radiotherapy or surgery) are used. Various objectives can be pursued, such as reducing side effects or improving tolerability, extending the treatment-free period or survival time, increasing cure rates or improving quality of life.
At the CCC Hannover, we offer our patients a wide range of clinical trials. For studies in the early phase of drug development, we work closely with the Clinical Research Center (CRC - Clinical Research Center) . Trial participants benefit from a qualified trial team that works closely with the specialist staff of the respective organ cancer center. Treatment takes place in the respective outpatient area and/or the modern wards at the CRC.
You can find a list of current trials at the CCC Lower Saxony and your region on the trial platform of the CCC Lower Saxony. If you have any questions about clinical trials or participation, please contact us atstudien-ccc@mh-hannover.de.
Questions about clinical trials
If you would like general information about clinical trials in cancer treatment or are thinking about participating in a clinical trial, you will find more detailed information below. The following information does not replace the necessary information that must be provided by the respective investigator when participating in trials.
Clinical studies are complex and cover a wide variety of research purposes. A basic distinction is made between interventional and non-interventional studies (NIS).
In interventional studies, the study participants receive an active form of treatment, i.e. an intervention planned in advance. This can be, for example, treatment with a new drug (AMG, e.g. immunotherapy) or medical device (MPG, e.g. implant). In such studies, new diagnostic and surgical procedures are also investigated or lifestyle interventions (e.g. nutrition) are tested.
In non-interventional studies (NIS), also known as observational studies, the effects of treatments are "observed" and documented. In other words, no direct "intervention" takes place. These include observational studies (AWB), where findings are collected on drugs that have already been admitted. For this purpose, patients are specifically "observed" under everyday conditions, e.g. with regard to application behavior or quality of life during therapy.
There are also registry studies in which no specific treatment is prescribed, but in which the clinical treatment data is recorded in order to analyze the course of the disease or the patient's care situation, among other things.
All clinical trials are part of a lengthy, carefully planned and controlled process in cancer research that is subject to strict legal regulations. The aim is to answer clinical-scientific questions and, above all, to improve the therapeutic results of a particular disease, which includes, for example, higher cure rates or lower rates of side effects.
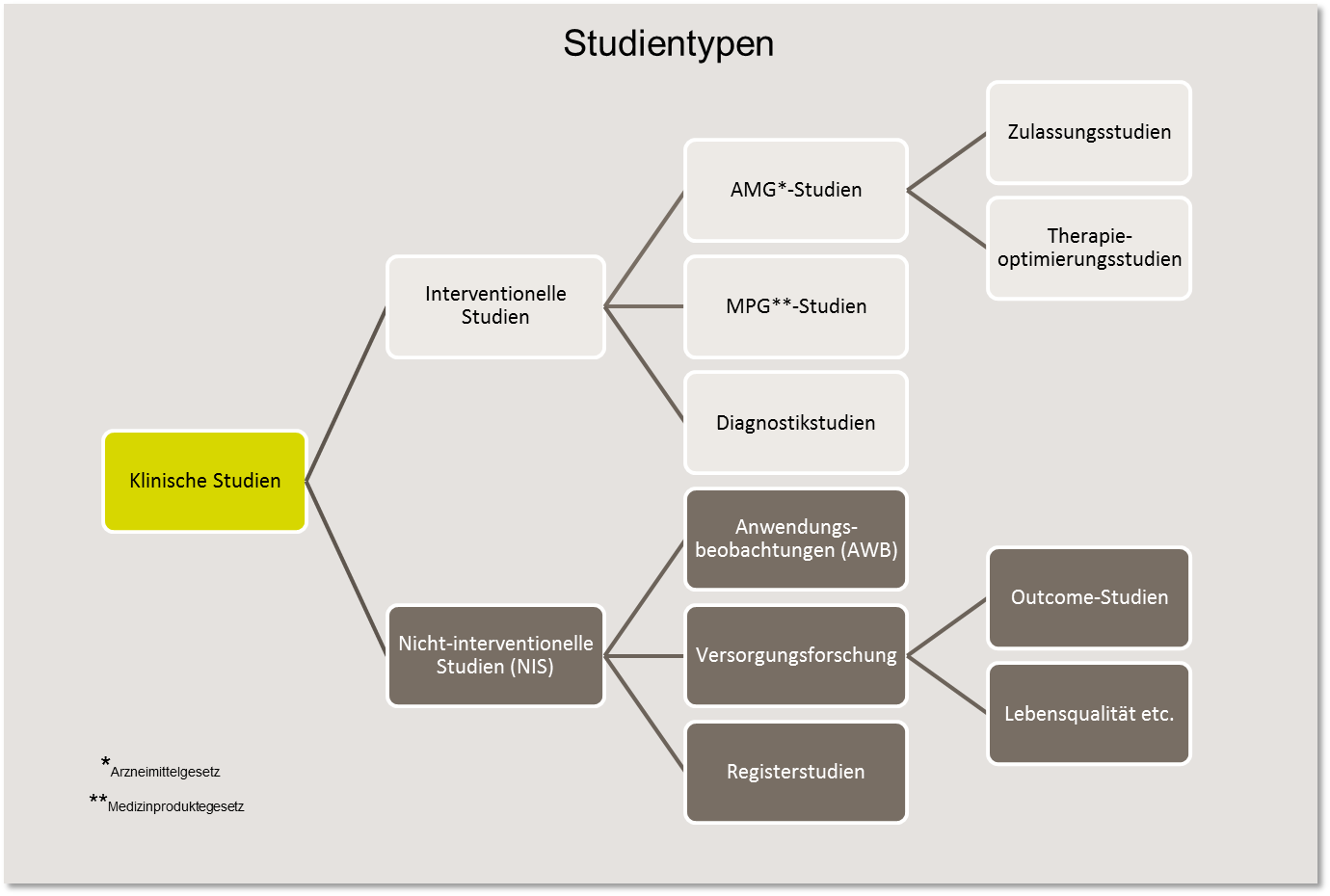
In clinical trials in cancer treatment, the study participants are usually patients with cancer. If they receive the study therapy as part of the treatment, it is a therapy study. If new forms of treatment (e.g. new drugs or their combinations with and without other therapeutic measures) are investigated, which enable the authorities to assess the safety and efficacy of the treatment, these are registration studies.
However, therapy studies can also be carried out as therapy optimization studies , which aim to continuously develop already proven therapy procedures for the benefit of patients in order to increase the chances of recovery and improve quality of life. This often involves using already approved drugs with proven efficacy and optimizing their dosage and timing.
- their dosage and timing,
- the combination with other medications
- or the use of additional forms of treatment (e.g. radiation, surgery, chemotherapy).
In contrast to the approval studies, the difference to standard treatment in therapy optimization studies is not very great.
In recent decades, clinical trials have led to significant advances in the diagnosis and treatment of cancer and have contributed to a significant improvement in the chances of survival and quality of life for most forms of cancer. Nevertheless, the therapy results for many treatment situations are still unsatisfactory and further improvement is necessary. This applies to newly diagnosed diseases that do not respond to standard treatment and, above all, to patients with metastases or relapses of the disease. In order to be able to use new treatment methods on a broad scale, they must be tested in clinical trials.
In order to carry out a therapy study, new forms of treatment must be evaluated in a controlled manner. To this end, the new treatments are statistically planned, systematically tested and carefully evaluated on a sufficiently large number of patients. This makes it possible to determine how effective and how safe the treatment methods really are. The benefits of the therapy can be convincingly demonstrated and possible risks identified.
The results of clinical studies help physicians to gain greater confidence in dealing with new treatment methods. Legal regulations and control mechanisms ensure that patient safety is guaranteed.
In Europe, there is a precisely defined procedure that must be followed before new forms of treatment are tested on patients. First, the active substance is developed and subjected to scientific laboratory tests (preclinical studies). Only when the results of the laboratory tests provide convincing data that support the benefits of a new drug may it be tested on patients in an orderly phased program. Therapy studies are the final step in the research process.
The entire process from drug development to market approval takes around 13.5 years on average. Of the 10,000 different substances that are initially considered as candidates for a new drug, 250 make it into the preclinical phase and only 5 into the clinical test phase.
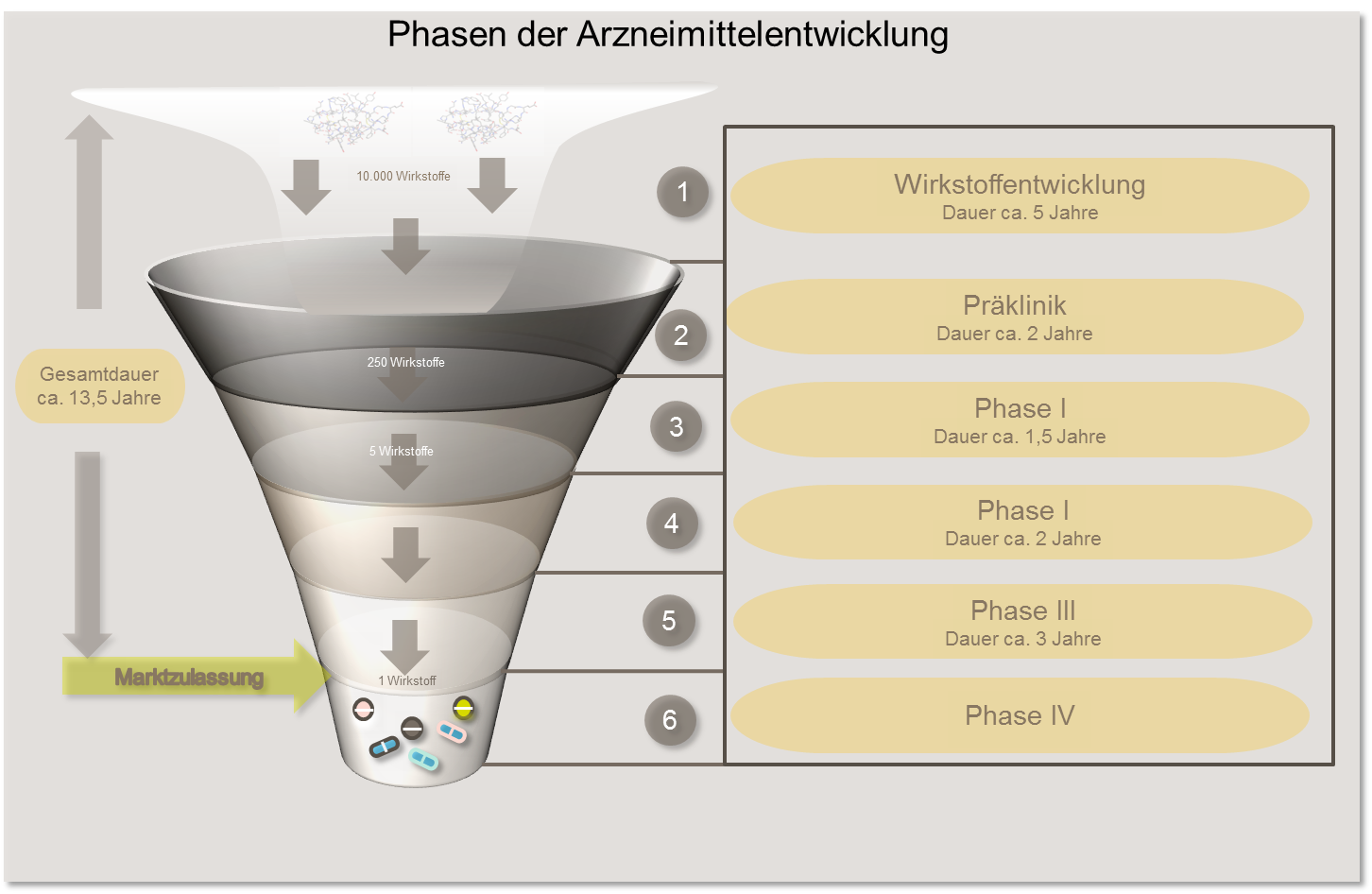
In phase I studies, a new therapeutic procedure that has shown promising results in preclinical studies is used for the first time in humans. The tolerability of the drug, its distribution and its breakdown in the body are initially investigated in a small group of patients. The dose that can be safely administered will be tested. As unexpected side effects cannot be completely ruled out, only patients for whom all standard treatment options have already been exhausted may take part in phase I trials. At the same time, however, there is the chance of being the first to gain access to a new, effective drug.

Based on the results of the phase I studies, the efficacy and appropriate dosage for certain forms of cancer, sometimes in combination with other drugs, is tested in a subsequent phase II. The aim is also to systematically record any side effects that occur.

Once the phase II studies have been completed and an optimal dosage has been determined, the aim of phase III is to provide reliable proof of the efficacy and safety of the drug under investigation. To this end, the new therapeutic procedure is compared with a known standard treatment. So-called randomized trials are often carried out (see "Methods"). Phase III trials require large numbers of patients , which makes it necessary to carry them out in a large number of treatment facilities (multicenter trials). Phase III trials serve as the basis for admission of the new treatment. Only when an advantage of the new therapy over standard treatments can be demonstrated can the new procedure be adopted into standard care.

In phase IV studies, the efficacy and safety of an already approved drug or procedure is investigated under real, everyday practical conditions. A very large number of participants are included in the studies. In contrast to phase III trials, most patients of different age groups and with various concomitant diseases are also allowed to participate. In this way, rare side effects and interactions with other medications can also be recorded. Phase IV trials are limited to the approved area of application and the approved dosage of the drug.

In order to ensure a high degree of reliability of the results obtained in a clinical study, various methods are used within a study. The required methods are already precisely defined during the planning of the study. In this way, external factors that could influence the results should be excluded as far as possible. The most important study methods include
Inclusion and exclusion criteria
Before the start of a study, clear parameters (e.g. type of cancer, stage of the disease, concomitant diseases, age or previous cancer therapies) are used to precisely describe which patients may participate in the study. These are referred to as inclusion criteria (characteristics that must be present) and exclusion criteria (characteristics that prevent participation in the study). The definition of these inclusion and exclusion criteria ensures that only data from patients with comparable disease characteristics are used to answer the study question. In addition, it should be avoided that patients are exposed to unacceptable burdens and risks when participating in the study (e.g. due to existing comorbidities).
Control group
To answer questions in clinical studies, it is often necessary to conduct the study in a controlled manner. This means that the participants are divided into several groups (treatment arms) with different treatments. Patients who receive the new treatment are in the experimental group. In the control group are those who receive either the proven standard treatment or a placebo. However, due to the severity of cancer, placebo-controlled studies are rare in cancer research. Instead, the standard therapy is usually used in the control group.
Randomization
In controlled trials, patients are often randomly assigned to a treatment group (randomized trials). This ensures the comparability of treatment results in the treatment arms and minimizes the risk of unequal distribution of patients. For example, it can be avoided that all patients of one gender or age are assigned to only one group.
Blinding
As expectations regarding the use of a new drug can change the study results, therapy studies are often conducted in a "blinded" manner. This means that patients only find out which drug they have received at the end of the treatment. Doctors' knowledge can also unconsciously influence the results of the study. For example, they could care more intensively for all those treated with the new drug than for the others. To rule out this effect, studies are often even double-blinded. This means that neither the patients nor the study staff conducting the trial know who belongs to which treatment group.
The safety of patients in clinical trials is already essential when planning a therapy study and is constantly monitored during the study.
International regulations and laws
Strict legal regulations and guidelines apply to all clinical trials in Germany and internationally (medicinal products: REGULATION (EU) 2014/536, medical devices: REGULATION (EU) 2017/745), to which all physicians and other persons who treat patients as part of clinical trials or participate in the development of clinical trials must adhere. In Germany, these are mainly regulated in the German Medicinal Products Act (AMG) and the Medical Devices Implementation Act (MPDG).
All members of the study team (physicians, study nurses, documentalists) who accompany patients during their participation in a therapy study must be specially trained and are obliged to act in accordance with the rules of "Good Clinical Practice" (GCP). These are internationally recognized rules for conducting clinical trials that are based on ethical and scientific principles. The focus here is on criteria to ensure the quality of the study results as well as the information and protection of study participants.
Review by Ethics Committee and authorities
Possible risks are strictly checked by ethics committees and authorities before the start of the study, who pay particular attention to the safety of the study participants. The study only receives a positive assessment if the legal rights and safety of patients are also safeguarded. Finally, approval from the competent higher federal authority (BfArM or Paul Ehrlich Institute) is required before new therapies can be used in standard care for all patients. The local authorities at state level regularly review the conduct of studies at the treatment center. Ethics committees and the authorities can withdraw their approval if it becomes apparent during the trial that patient safety is not guaranteed.
Subject insurance
Insurance for study participants (subject insurance) must be taken out for every clinical trial in order to cover any financial losses (e.g. financial risk due to damage to health) that may occur for patients as a result of the trial therapy.
Monitoring patient safety during the clinical trial
During regular follow-up appointments, certain safety parameters are checked (e.g. by taking blood samples) and any side effects are documented. The ethics committee and authorities are informed about the progress of the study and can take appropriate measures if side effects occur, such as adapting the study protocol or discontinuing the entire study. It is therefore necessary to attend all scheduled appointments within the study. Only in this way can all health changes and abnormalities be recorded.
In the event of side effects or discomfort during a study, the patient has the opportunity to contact the responsible study team at any time in order to receive immediate help.
Study protocol
Every study has a medical study director who is responsible for planning, conducting and evaluating the study. A study protocol, which must be reviewed by the Ethics Committee and authorities before the study begins, usually describes
- The rationale and objectives of the study
- Study procedure and duration
- Number of patients to be treated
- Inclusion and exclusion criteria
- Description of the study therapy and, if applicable, the different therapy arms
- List of necessary examinations
- Information on expected side effects
- Data to be documented
- Patient information
- Data Protection measures
- Statistical evaluation
All physicians participating in the particular study use the same protocol and ensure that all patients within the study are treated in the same way and that the results obtained are analyzable and comparable. All members of a trial team must undergo regular special training in order to be admitted to work in clinical trials.
Patient information and consent
Before being included in a trial, the patient concerned must be informed in detail, both verbally and in writing, about all aspects of the trial, foreseeable risks, advantages and disadvantages and other treatment options. The patient's legal rights and obligations as a participant in a therapy study are also explained and the confidential treatment of patient data is assured.
The patient is given sufficient time to think about the decision to participate. Only if the patient agrees to participate in the study on the basis of comprehensive information and confirms this with a signature can he or she be treated in a therapy study. If the patient does not agree, the treatment team will offer a treatment option in accordance with current standards. Without consent, the conduct of any study activities by the study staff is prohibited.
Procedure for the patient
Clinical studies are usually conducted at specialized Clinical Departments or in specialist practices, as specialized and specially trained personnel are required. Doctors, nurses and study staff ensure that the regulations of the study protocol are followed as closely as possible and that the course of therapy, all test results and possible side effects are continuously documented in writing.
After the written and verbal explanation by the study physician, the subsequent consent to participate and the examination of all inclusion and exclusion criteria, enrollment (inclusion) in the study can take place. During the course of the study, the study participants are treated and examined at precisely defined times (visits) and according to a fixed schedule.
An initial/preliminary examination is often carried out first, during which the patient's baseline values (e.g. blood values or body weight) are recorded in order to provide a basis for later comparison with the values after treatment. Subsequently, the visits are often very frequent and additional visits to the physician and examinations are required. These primarily serve to better monitor the course of treatment and are therefore ultimately also in the interests of the patient. The number and type of examinations, including follow-up checks and patient surveys on well-being or treatment success, are specified in the study protocol and are explained to the patient before participation in the study.
Even after the actual treatment, there are usually further follow-up appointments for monitoring and questioning for a fixed period of time. The last regular visit, which takes place according to the study protocol, is referred to as the final visit. This is the last time the patient's values are recorded. After this, the study is officially over for the participant. However, many studies offer patients regular follow-up examinations even after the official end.
Study patients have the opportunity to find out about the progress of the study at any time. International databases, in which every clinical trial must be registered, are used for this purpose. As participation in the study is voluntary, patients can also end their participation at any time and without giving reasons, without this having any disadvantages for their further treatment. In this case, however, patients should urgently consult with the treating study physician and not simply discontinue treatment. This is also for their own safety in such a case.
The decision to participate in a study often has to be made at a time when the patient concerned is under particular pressure due to the diagnosis and their state of health. In many cases, treatment must be started quickly in order to push back the disease. Nevertheless, it is necessary to make use of all available information and support and to make the decision yourself after careful consideration.
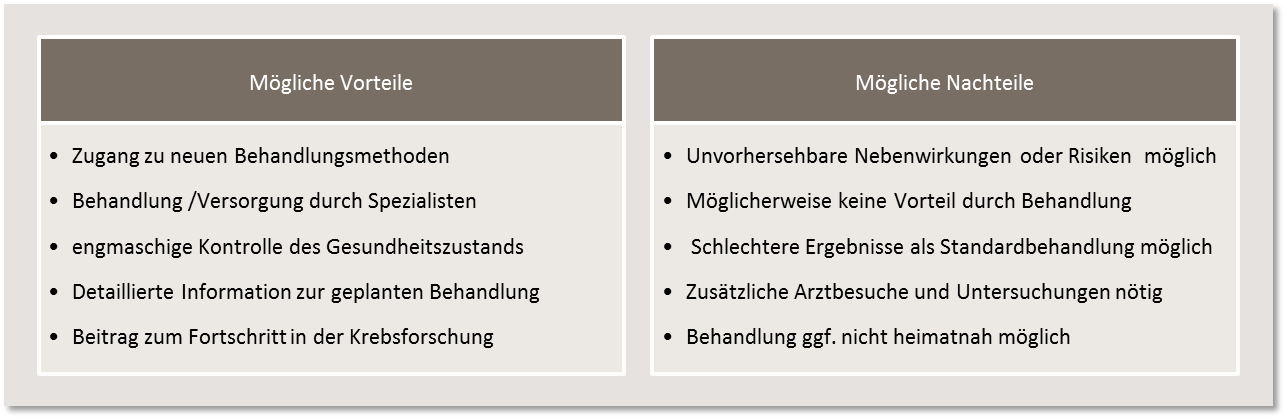
The decision to participate in a clinical trial lies exclusively with the patient concerned. A decision not to participate has no negative impact on regular treatment. This is carried out in accordance with existing standards. When deciding for or against participation in a trial, possible advantages and disadvantages must also be weighed up: