Secondary treatment of breast cancer in plastic surgery
In addition to investigating clinical aspects (see menu item Clinical Research), the working group is also studying the effects of the oestrogen receptor antagonist tamoxifen on stem cells and healthy mammary epithelial cells. Here, the change in gene expression in stem cells and healthy epithelial cells under the influence of tamoxifen is examined in more detail.
Scientific management
Dr. rer. biol. hum. Vesna Bucan
Phone: 0511 532 - 8788
New antimicrobial peptides as anti-cancer therapeutics
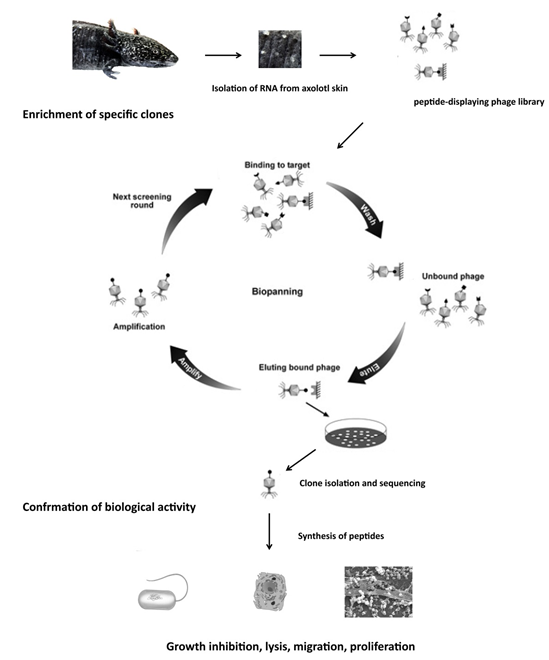
With over seven million deaths per year and rising morbidity, cancer is one of the most common human diseases. Traditional therapies are associated with severe side effects, and advanced stages of the disease are usually incurable. In addition, the number of therapy-resistant cancer cells is increasing. In the search for new effective cancer therapeutics with fewer side effects, scientists are increasingly focusing on so-called antimicrobial peptides (AMP). Some of these peptides are not only effective against various pathogens but also have an effect against cancer cells.
Our research group is working on the identification of peptides with antimicrobial activity from axolotl skin secretions as potential novel antibiotics for Human medicine and cancer therapy.
We investigate the mechanism of action of AMPs in terms of speed of action, preferred binding structures, peptide-peptide interaction and selectivity for cancer cells.
Scientific Director
Dr. rer. nat. Sarah Strauß, Head of Laboratory
Kerstin Reimers Laboratory for Regenerative Biology
Phone: +49 511 532-8863
mobile: +49 1761 532-8790
Molecular biology of breast cancer
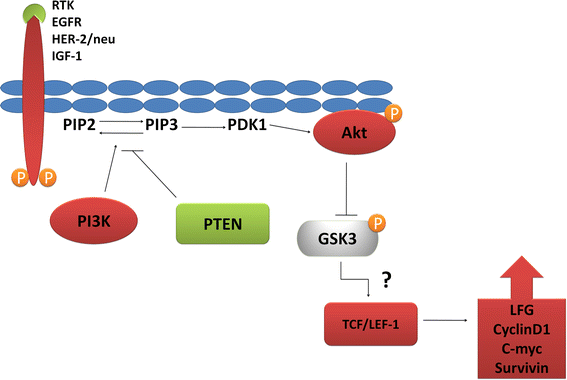
Evaluation of a concept for antitumor therapy by blocking the expression of apoptosis-inhibiting proteins through LFG-RNA interference
The Lifeguard (LFG) protein is frequently found in many tumors, especially in breast cancer. It protects the cells from self-destruction and prevents the controlled elimination of defective body cells. This promotes the growth of tumor cells and metastases. If LFG is switched off, significantly more cancer cells die under chemotherapy than with functional protein. Trastuzumab and erlotinib have already been successfully tested in combination with the elimination of LFG in cell culture. However, what has been so impressively successful in cell cultures must now be proven in a living organism.
Previous projects on the role of LFG in breast cancer and the identification of the pathway have been funded by the Claudia von Schilling Foundation, the Lower Saxony Cancer Society and the Gottfried Arndt Foundation.
Scientific management
Dr. rer. hum. biol. Vesna Bućto
Kerstin Reimers Laboratory for Regenerative Biology
Phone: +49 511 532-8788,
mobile: +49 1761 532-8803
Further reading
Maurer, V., Reimers, K., Lück, H. J., Vogt, P. M., & Bucan, V. (2017). Anti-apoptotic protein Lifeguard does not act as a tumor marker in breast cancer. Oncology Letters, 13(3), 1518-1524.
Müller, J., Maurer, V., Reimers, K., Vogt, P. M., & Bucan, V. (2015). TRIM21, a negative modulator of LFG in breast carcinoma MDA-MB-231 cells in vitro. International journal of oncology, 47(5), 1634-1646.
Dastagir, N., Lazaridis, A., Dastagir, K., Reimers, K., Vogt, P. M., & Bucan, V. (2014). Role of lifeguard β-isoform in the development of breast cancer. Oncology reports, 32(4), 1335-1340.
Bucan, V., Choi, C. Y., Lazaridis, A., Vogt, P. M., & Reimers, K. (2011). Silencing of anti-apoptotic transmembrane protein lifeguard sensitizes solid tumor cell lines MCF-7 and SW872 to perifosine-induced cell death activation. Oncology letters, 2(3), 419-422.
Bucan, V., Reimers, K., Choi, C. Y., Eddy, M. T., & Vogt, P. M. (2010). The anti-apoptotic protein lifeguard is expressed in breast cancer cells and tissues. Cellular & molecular biology letters, 15(2), 296-310.
Reimers, K., Choi, C. Y., Bucan, V., & Vogt, P. M. (2008). The Bax Inhibitor-1 (BI-1) family in apoptosis and tumorigenesis. Current molecular medicine, 8(2), 148-156.
Multifunctional iron oxide-gold nanohybrids - A theranostic concept in breast cancer
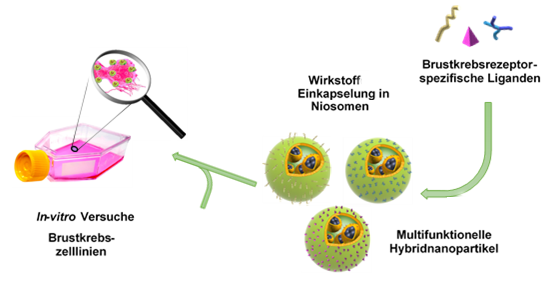
Despite its high potential in breast cancer treatment, the clinical use of hyperthermia therapy is currently limited due to the inhomogeneous and uncontrolled heating of the entire tumor tissue. The working group is therefore working on the development of a novel theranostic hybrid nanoparticle system that enables optical diagnostics and efficient thermotherapy for breast carcinomas.
In cooperation with the Technical University of Braunschweig and the Physikalisch-Technische Bundesanstalt (PTB), biocompatible iron oxide-gold nanohybrids are synthesized, functionalized and characterized in order to establish a hierarchical and tumor-specific structure that allows visual detection of tumor cells. Furthermore, the heat generation efficiency is investigated by coupled excitation under near-infrared irradiation and an alternating magnetic field using in-vitro and in-vivo experiments. The aim is to develop an innovative strategy for the production and application of theranostic agents in order to overcome the current problems of low specificity and uncontrolled heating and to optimize the promising concept of breast cancer hyperthermia.
Project leader:
Dr. rer. hum. biol. Vesna Bućan
Phone: +49 511 532-8788,
mobile: +49 1761 532-8803