Research in the women's clinic
The Women's Clinic is represented by three working groups in the research center and also carries out other research projects.
Women's Clinic at the Research Center
Head: Dr. rer. nat. Thilo Dörk-Bousset
Phone: 0511/532-6075 (office)
Phone: 0511/532-6077 (laboratory)
Fax: -6081
E-mail: doerk.thilo@mh-hannover.de
Area: MHH - OU 6411 - Building I11, Level S0.
AG Molecular Gynecology
Dr. rer. nat. Thilo Dörk-Bousset
AG Biochemistry and Tumor BiologyProf. Dr. Dr. Ralf Hass
AG Reproductive Medicine and Molecular PerinatologyProf. Dr. med. Frauke von Versen-Höynck
Further research projects at the Women's Clinic
Scientific focus:
- Evaluation of various test methods for the diagnosis of genital HPV infection
- Photodynamic diagnosis and therapy of HPV-associated dysplasia of the cervix and vulva
- Prevention of HPV infection through vaccination
- Health economic studies on HPV-associated dysplasia / carcinomas
Contact:
Clinical Department of Gynecology and Obstetrics
Univ.-Prof. Dr. med. med. Peter Hillemanns
Tel.: 0511-532-6144
Fax: 0511-532-6145
Contributors:
Women's Clinic at the MHH Research Center
Prof. Dr. rer. nat. Ralf Hass
Dr. rer. nat. Thilo Dörk-Bousset
Ongoing research projects:
- HPV vaccination studies [HPV 070]
- Self-examination methods for HPV smear tests in the context of cervical cancer prevention [HaSCo]
- Genetic disposition for dysplasia and carcinoma of the cervix [ZerviGen] (see Molecular Gynecology Working Group of the Research Center)
- Comparison of different HPV test systems
- Photodynamic therapy of CIN 2 - 3 [Cevira]
- HPV detection from urine
- Therapeutic HPV 16 vaccination for CIN 2/3 [VB C-01]
- Therapeutic HPV 16 vaccination and checkpoint inhibition in recurrent cervical carcinoma [VB C-02] (see clinicaltrails and Company Announcement incl. graphic)
- Cervical cancer and sentinel. International phase 3 study [SENTICOL III] (see PubMed)
- Colposcopy study group
Third-party funding in the last five years:
- German Cancer Society (DKH)
- German Research Foundation (DFG)
- Federal Ministry of Education and Research (BMBF)
- State of Lower Saxony
- Volkswagen Foundation
- Wilhelm Sander, Jöster, Bartling Foundation
- Novartis Institute of Biomedical Research
- German Alliance for Global Health Research
Scientific publications of the last five years:
Items 1-15 of 15(Display the 15 citations in PubMed)
Hillemanns P, Denecke A, Woelber L, Böhmer G, Jentschke M, Schjetne KW, Bruins Slot KMH, Fredriksen AB. Clin Cancer Res. 2022 Sep 21:CCR-22-1927. doi: 10.1158/1078-0432.CCR-22-1927. online ahead of print. PMID: 36129459
Vink FJ, Meijer CJLM, Hesselink AT, Floore AN, Lissenberg-Witte BI, Bonde JH, Pedersen H, Cuschieri K, Bhatia R, Poljak M, Oštrbenk Valenčak A, Hillemanns P, Quint WGV, Del Pino M, Kenter GG, Steenbergen RDM, Heideman DAM, Bleeker MCG. Clin Infect Dis. 2022 Jun 10:ciac433. doi: 10.1093/cid/ciac433. Online ahead of print. PMID: 35686306
Chen F, Novák Z, Dannecker C, Mokráš C, Sui L, Zhang Y, You Z, Han L, Lang J, Hillemanns P. BMJ Open. 2022 Jun 6;12(6):e061740. doi: 10.1136/bmjopen-2022-061740. PMID: 35667715 Free PMC article.
Hampl M, Hesselink AT, Meijer CJLM, Denecke A, Einhorn I, Reinecke-Luethge A, Geppert CI, Jentschke M; Members of the German Study Group of Colposcopy (SGK), Petry KU, Hillemanns P. Int J Cancer. 2022 Nov 1;151(9):1578-1585. doi: 10.1002/ijc.34153. Epub 2022 Jun 20. PMID: 35666529
Beckmann MW, Stübs FA, Koch MC, Mallmann P, Dannecker C, Dietl A, Sevnina A, Mergel F, Lotz L, Hack CC, Ehret A, Gantert D, Martignoni F, Cieslik JP, Menke J, Ortmann O, Stromberger C, Oechsle K, Hornemann B, Mumm F, Grimm C, Sturdza A, Wight E, Loessl K, Golatta M, Hagen V, Dauelsberg T, Diel I, Münstedt K, Merz E, Vordermark D, Lindel K, Wittekind C, Küppers V, Lellé R, Neis K, Griesser H, Pöschel B, Steiner M, Freitag U, Gilster T, Schmittel A, Friedrich M, Haase H, Gebhardt M, Kiesel L, Reinhardt M, Kreißl M, Kloke M, Horn LC, Wiedemann R, Marnitz S, Letsch A, Zraik I, Mangold B, Möckel J, Alt C, Wimberger P, Hillemanns P, Paradies K, Mustea A, Denschlag D, Henscher U, Tholen R, Wesselmann S, Fehm T. Obstetrics Gynecology. 2022 Feb 11;82(2):139-180. doi: 10.1055/a-1671-2158. eCollection 2022 Feb. PMID: 35169387 Free PMC article.
Ramachandran D, Dennis J, Fachal L, Schürmann P, Bousset K, Hülse F, Mao Q, Wang Y, Jentschke M, Böhmer G, Strauß HG, Hirchenhain C, Schmidmayr M, Müller F, Runnebaum I, Hein A, Stübs F, Koch M, Ruebner M, Beckmann MW, Fasching PA, Luyten A, Dürst M, Hillemanns P, Easton DF, Dörk T. Hum Mol Genet. 2022 Aug 17;31(15):2483-2497. doi: 10.1093/hmg/ddac031. PMID: 35157032 Free PMC article.
7 CoCoss-Trial: Concurrent Comparison of Self-Sampling Devices for HPV-Detection .
Ertik FC, Kampers J, Hülse F, Stolte C, Böhmer G, Hillemanns P, Jentschke M. Int J Environ Res Public Health. 2021 Oct 2;18(19):10388. doi: 10.3390/ijerph181910388. PMID: 34639688 Free PMC article.
8. association of preoperative cone biopsy with recurrences after radical hysterectomy.
Klapdor R, Hertel H, Delebinski L, Hillemanns P. Arch Gynecol Obstet. 2022 Jan;305(1):215-222. doi: 10.1007/s00404-021-06145-0. Epub 2021 Jul 21. PMID: 34291339 Free PMC article.
Kampers J, Gerhardt E, Sibbertsen P, Flock T, Klapdor R, Hertel H, Jentschke M, Hillemanns P. Arch Gynecol Obstet. 2021 Sep;304(3):577-587. doi: 10.1007/s00404-021-06082-y. Epub 2021 May 22. PMID: 34021804 Free PMC article. Review.
10. prophylactic HPV vaccination after conization: A systematic review and meta-analysis .
Jentschke M, Kampers J, Becker J, Sibbertsen P, Hillemanns P. Vaccine. 2020 Sep 22;38(41):6402-6409. doi: 10.1016/j.vaccine.2020.07.055. Epub 2020 Aug 4. PMID: 32762871 Review.
Hillemanns P, Tempfer C, Beckmann MW, Küppers V, Quaas J. Obstetrics Gynecology. 2020 Aug;80(8):809-812. doi: 10.1055/a-1193-5136. Epub 2020 Aug 14. PMID: 32817987 Free PMC article.
12. association of genomic variants at PAX8 and PBX2 with cervical cancer risk.
Ramachandran D, Wang Y, Schürmann P, Hülse F, Mao Q, Jentschke M, Böhmer G, Strauß HG, Hirchenhain C, Schmidmayr M, Müller F, Runnebaum I, Hein A, Koch M, Ruebner M, Beckmann MW, Fasching PA, Luyten A, Dürst M, Hillemanns P, Dörk T. Int J Cancer. 2021 Apr 27. doi: 10.1002/ijc.33614. Online ahead of print. PMID: 33905146 Free article.
Klischke L, von Ehr J, Kohls F, Kampers J, Hülse F, Schmitz M, Hennig A, Dörk T, Hillemanns P, Jentschke M. J Virol Methods. 2021 Sep;295:114219. doi: 10.1016/j.jviromet.2021.114219. Epub 2021 Jun 24. PMID: 34175345
Cibula D, Dostalek L, Hillemanns P, Scambia G, Jarkovsky J, Persson J, Raspagliesi F, Novak Z, Jaeger A, Capilna ME, Weinberger V, Klat J, Schmidt RL, Lopez A, Scibilia G, Pareja R, Kucukmetin A, Kreitner L, El-Balat A, Pereira GJR, Laufhütte S, Isla-Ortiz D, Toptas T, Gil-Ibanez B, Vergote I, Runnenbaum I. Eur J Cancer. 2021 Jan;143:88-100. doi: 10.1016/j.ejca.2020.10.037. Epub 2020 Dec 5. PMID: 33290995
15 Psychological distress in cervical cancer screening: results from a German online survey.
Jentschke M, Lehmann R, Drews N, Hansel A, Schmitz M, Hillemanns P. Arch Gynecol Obstet. 2020 Sep;302(3):699-705. doi: 10.1007/s00404-020-05661-9. Epub 2020 Jun 27. PMID: 32594298 Free PMC article.
Main areas of research:
- Chimeric antigen receptors against gynecological carcinomas
- NK cell therapy
- Adoptive immunotherapy against ovarian carcinoma
- Adoptive immunotherapy against cervical carcinoma
- Adoptive immunotherapy against breast carcinoma
- Specific cell therapy against cancer stem cells
Description:
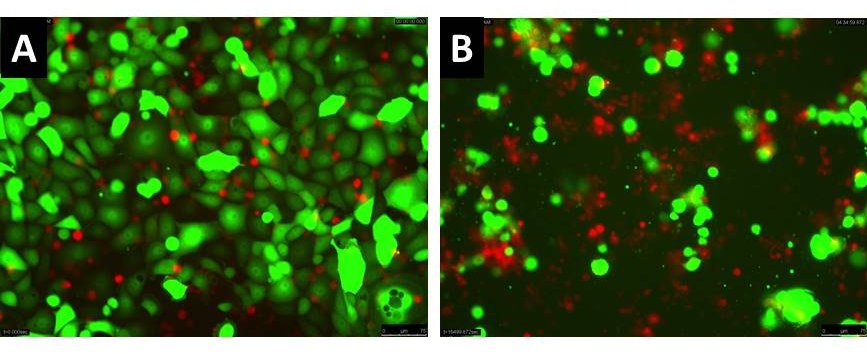
The immune system protects us not only from foreign infections but also from our own malignant cells, thus preventing the development of cancer on a daily basis. In some cases, however, this guardian function is not sufficient and ovarian cancer, for example, can develop. Previous treatment methods such as extensive surgery and chemotherapy are unfortunately not successful in all cases and are sometimes associated with severe side effects. Ideally, the immune system should be retrained to recognize and eliminate cancer cells on its own. One possible strategy for this is to equip immune cells, such as T or natural killer (NK) cells, with special receptors that can specifically recognize the tumour cells. The special receptors we use are called chimeric antigen receptors (CAR). We have already produced various receptors that sensitize non-specific immune cells (NK cells) to the tumour cells. The photo shows an experiment in which ovarian cancer cells (green) were combined with NK cells specified by us (red). Picture A shows the initial state. Image B after a few hours. Here it can be seen that practically all green cancer cells have been destroyed. In our experiments, we were able to show that we can not only eliminate various ovarian cancer cells in a targeted manner, but also specifically attack cancer stem cells, which are characterized by being less sensitive to chemotherapy. This approach in particular may be a future therapy for ovarian cancer, which cannot be treated well with chemotherapy. These strategies are currently being tested in further trials so that they can ideally be used as a therapy for patients in a few years' time.
Contact:
Prof. Dr. med. Rüdiger Klapdor
E-mail: klapdor.ruediger@mh-hannover.de
Cooperations:
- Women's Clinic at the Research Center, MHH
- Institute of Experimental Haematology, MHH
Scientific publications:
Items 1-3 of 3(Display the 3 citations in PubMed)
1. characterization of a Novel Third-Generation Anti-CD24-CAR against Ovarian Cancer.
Klapdor R, Wang S, Morgan M, Dörk T, Hacker U, Hillemanns P, Büning H, Schambach A. Int J Mol Sci. 2019 Feb 3;20(3):660. doi: 10.3390/ijms20030660. PMID: 30717444 Free PMC article.
Klapdor R, Wang S, Hacker U, Büning H, Morgan M, Dörk T, Hillemanns P, Schambach A. Hum Gene Ther. 2017 Oct;28(10):886-896. doi: 10.1089/hum.2017.168. Epub 2017 Aug 24. PMID: 28836469
Klapdor R, Wang S, Morgan MA, Zimmermann K, Hachenberg J, Büning H, Dörk T, Hillemanns P, Schambach A. Biomedicines. 2021 Sep 28;9(10):1339. doi: 10.3390/biomedicines9101339. PMID: 34680456 Free PMC article.
The Research Obstetric Biobank of Obstetrics & Prenatal Medicine aims to preserve pregnancy products in a suitable form for scientific questions and is part of the MHH biobank.
The primary aim is to enter into individual research collaborations within and outside the Women's Clinic and to provide access to interdisciplinary collaborations for employees of the Women's Clinic who are interested in science.
The application form for products can be found in the attached PDF file.
The transfer of samples is associated with a contribution towards expenses.
The current collaborations that are supplied with products are listed below.
Responsible for the Research Obstetric Biobank:
Univ.Prof. Dr. med. C.S. von Kaisenberg
Head of the Department of Obstetrics and Prenatal Medicine
DEGUM III, Diploma in Fetal Medicine (FMF UK)
Clinical Department of Gynecology, Obstetrics and Reproductive Medicine
Hannover Medical School
Carl-Neuberg-Str. 1
D-30625 Hannover
fon: +49 (0) 511 532-0, +49 (0) 176 1532 3454
fax: +49 (0) 511 532 8004
e-mail: vonkaisenberg.constantin@mh-hannover.de
- A Research Obstetric Biobank: Structure and Responsibilities pdf-file
Scientific cooperations
Cooperations within the Department of Obstetrics and Gynecology
- Prof. Hass, Biochemistry and Tumor Biology Group (0511 532 6070)
Expansion of umbilical cord derived mesenchymal stem cells with regards to GMP-conform production process. Prof. Hass, PD Dr. Kasper (0511 762 2967).
Characterization of subpopulations of umbilical cord mesenchymal stem cells under normoxic and hypoxic conditions. PhD student Niendorf. - Prof. Dr. von Versen-Höynck, WG Experimental Obstetrics (0511 532 6074)
The role of adenosine in placental development and placental amino acid transport Contact: Prof. Dr. von Versen-Höynck / Natallia Darashchonak , Ethikvotum MHH No. 3254, 10/2009-9/2012, approx. 1-2 placentas per week.
The role of vitamin D in placental developmental processes (pilot study)
Contact: Prof. Dr. von Versen-Höynck, Ethikvotum MHH No. 3254 , 10/2010-10/2012, maternal and umbilical cord blood (pre-eclampsia, normal controls), approx. 10ml blood 1-2 x / week.
MicroRNA's in pre-eclampsia and HELLP (pilot study)
Prof. Dr. Dr. von Versen-Höynck, Ethikvotum MHH No. 3254 , 10/2010-10/2012, maternal and umbilical cord blood (pre-eclampsia, normal controls), approx. 10ml blood 1-2 x / week. MicroRNA's in pre-eclampsia and HELLP (pilot study) Prof. Dr. Dr. Dr. von Versen-Höynck, Ethikvotum MHH No. 3254 , 10/2010-10/2012, maternal blood prenatal, approx. 10ml blood, 8 pre-eclampsia, 8 HELLP, 16 normal patients.
Maternal PKU': In vitro studies on placental amino acid transport and metabolism (cooperation pediatrics)
Prof. Das, Clinical Department of Pediatrics, PhD student Kristin Goedecke, ethics vote MHH No. 3254 , start: 10/2010-10/2012, 1x placenta / week.
Studies on the role of vitamin D in endothelial repair processes (cooperation pediatrics)
Prof. Das, Clinical Department of Pediatrics, StrucMed Program, Ethikvotum MHH No. 3254 , 08/2011-08/2012 , 1-2 umbilical cords / week.
Chronic hypoxia of the umbilical cord: effect on aerobic energy supply, in particular regulation of mitochondrial ATP synthase in HUVEC (Cooperation Pediatrics)
Prof. Das, Clinical Department of Pediatrics, StrucMed Program, Ethikvotum MHH No. 3254 , 08/2011-08/2012.
MHH-internal cooperations
- Prof. Schambach working group, Experimental Hematology, Hans Borst Center for Heart and Stem Cell Research (0511 532 6067)
Development of a standard protocol for ex vivo expansion of hematopoietic stem cells (HSCs) from umbilical cord blood, five subprojects in CB-HERMES (Cord Blood-Hematopoietic stem cells: Reliable Methods for ex-vivo ExpanSion).Dr. Schiedlmeier (0511 532 5134), Dr. Lachmann (0511 532 5266) Ethics vote MHH 869, samples: 3 x weekly
Umbilical cord blood (EDTA), stored at 4°C, isolation of CD34 cells by MACS
'Generation of human hematopoietic malignancies with CRISPR/cas9'
Contact Dr. Dr. Schwarzer, Tel: 5143, would like umbilical cord blood, start: 15.6.2015, duration: initially unlimited, full scientific cooperation.
Clonality of mesenchymal stem cell cultures from umbilical cord. Contact: Dr. Rothe: Tel. 0511 532 5137, start: immediately, until further notice, full scientific cooperation. - Working group Prof. Martin, Rebirth II (0511 532-8820)
Induction of pluripotent stem cells from young versus aged somatic cells: differences in reprogramming rates, karyotypic abnormalities and frequency of accumulated mutations. PhD student: Katarzyna Osetek (532-8658), PostDoc: Dr. Alexandra Haase (532-8820), Ethics vote: not necessary if samples anonymous, Start: 2011, Samples: fresh heparinized umbilical cord blood, fresh umbilical cords, irregular, report each time.
Development of a biohybrid lung: endothelialization of polymeric gas exchange membranes.
Dr. Michael Pflaum, LEBAO (532 8873), ethics vote: not necessary if samples anonymous, start: 2011, samples: fresh heparinized umbilical cord blood, fresh umbilical cords, irregularly, reports. - Working group Prof. Hansen, Clinical Department of Paediatric Pneumology, Allergology and Neonatology (0511 532 9138)
Neonatal B cells: functional and developmental characterization of human B cell subpopulations, Prof. Dr. Meyer-Bahlburg, (0511 532 6065), Ethikvotum MHH No: 517, Start: 2011, Samples: fresh unfrozen umbilical cord blood, irregular, reports.
Characterization of immunological tolerance states in the neonatal immune system. Prof. Dorothee Viemann (0511 532 7823, 17 3960), Dr. Sabine Pirr (0511 532 8895, 17 7061), Dr. Judith Friesenhagen (9785), Andrea Weitz (7824), Ethikvotum MHH No. 6031, start 2011, inclusion criteria: Newborn and premature infants, exclusion criteria: Malformation syndromes, connatal systemic diseases, samples Mon.-Thurs.: umbilical cord blood 10ml EDTA, 2ml serum, 30ml heparin blood, maternal blood 1ml EDTA, 2ml serum, 20ml heparin blood, store EDTA & serum in refrigerator, store heparin samples at room temperature. - Dr. Kirsch working group
Analysis of Intercellular Contacts in Primary Endothelial Cells. Svjetlana Lovric, Michaela Beese, Kristin Wyss, Johanna Kegel (0511 532 9357), MHH Ethikvotum No. 4344, Start: 2011, Samples: Umbilical cord, report. - Prof. Reinhardt working group
Acute myeloid leukemia and trisomy 21, Prof. Dr. Klusmann (0511 532 3252, 0175 5223683), MHH Ethics vote No. xxx, start: 2011, samples: Umbilical cord blood / umbilical cord of trisomy 21 newborns, must be informed. - Prof. Melk working group
Endothelial cellular senescence- a new target for vascular protection?
Investigation of INK4a/ARF expression in SNP variants of the CDNK2A/B gene locus associated with cardiovascular risk.
Prof. Dr. Hömme (0511 532 5597, -9507), MHH Ethikvotum 575, start: 2009, samples: Umbilical cord, on request. - Prof. Petri, Neurology
Therapeutic potential of human umbilical cord blood cells in amyotrophic lateral sclerosis (ALS)
Contact: Susanne Petri, (0511 532 3740), MHH Ethikvotum 3037, start 2012, umbilical cord blood, on request
Studies on the regeneration of immune cells after adult hematopoietic stem cell transplantation. Prof. Krueger, Regenerative Immunology, Jonas Blume 532 9738, Ethikvotum MHH 2454-2014, umbilical cord blood, much, for stem cell isolation, may have lain overnight, start: 15.5.2015
International cooperations
- Working Group Obstetrics and Prenatal Medicine, Prof. C. S. v. Kaisenberg
Prognosis Trial (Roche) for the prediction of pre-eclampsia (Prof. Dr. C.S. v. Kaisenberg - vonkaisenberg.constantin@mh-hannover.de), Dr. Jentschke)