G-LACC study
German-funded Laparoscopic Approach to Cervical Cancer

Welcome!
The G-LACC trial is a surgical non-inferiority trial with the aim of improving the treatment of cervical cancer. The standard treatment for this type of cancer is an abdominal radical/simple hysterectomy (ARH), in which the uterus is removed through a complex operation via an abdominal incision. The G-LACC study will compare this standard treatment with laparoscopic radical/simple hysterectomy (LRH) to find out whether one method is superior to the other. LRH is far less invasive than ARH and involves laparoscopy rather than surgery - for patients, this means less blood loss, a shorter hospital stay and a lower risk of post-operative complications than with open surgery. In addition, LRH can be supported by a surgical robot, which allows a more precise procedure.
The G-LACC study will start in 2024 and is open to all hospitals that meet the quality requirements.
Univ.-Prof. Dr. P. Hillemanns
Director of the Clinical Department of Gynaecology and Obstetrics
Responsible Investigator
Inclusion criteria
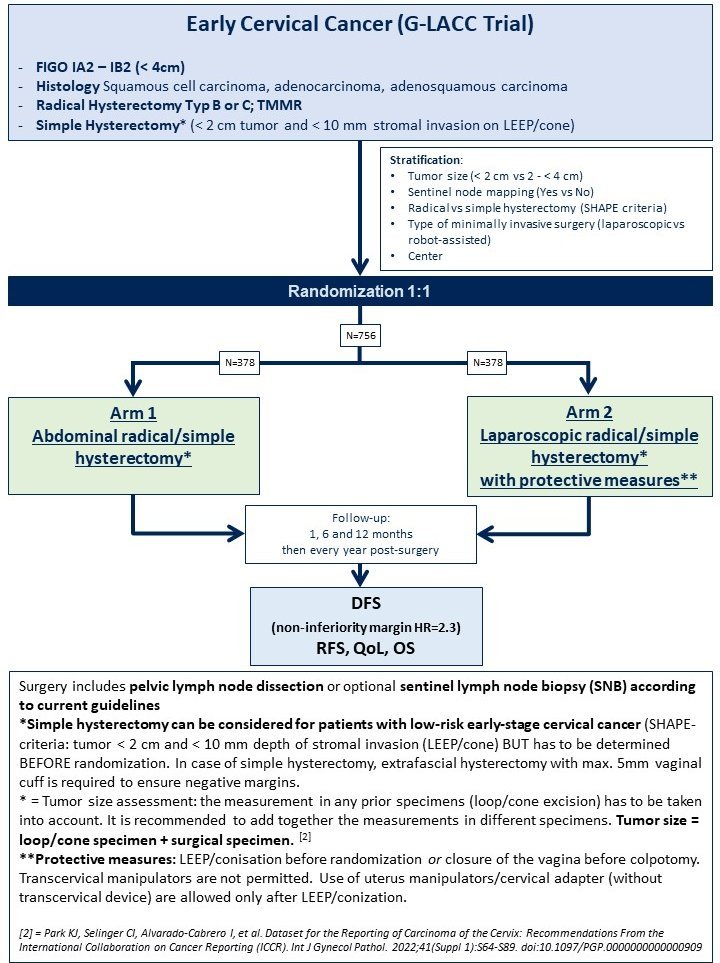
The G-LACC study is a surgical, prospective, multicenter, open-label, randomized, non-inferiority trial and is aimed at women with early-stage cervical cancer.
Women with early-stage cervical cancer (FIGO IA2 or IB1, tumor size ≤ 4cm) from up to 20-30 certified oncology centers throughout Germany are included.

- Histologically confirmed primary squamous cell carcinoma, adenocarcinoma or mixed type of cervix uteri
- FIGO stage IA2, IB1 or IB2
- Tumor size < 4 cm
- Planned radical hysterectomy according to Piver II or III or according to type B or C (Querleu and Morrow classification) or total mesometrial resection (TMMR)
OR:
Simple hysterectomy in patients with low-risk early stage cervical cancer (according to the SHAPE criteria* : with a tumour size < 2 cm and < 10 mm stromal invasion (LEEP/conization)). In the case of a simple hysterectomy, an extrafascial hysterectomy with a max. 5 mm vaginal cuff is required. - ECOG 0-1
- Signed informed consent form
- Women ≥ 18 years
* = Plante M, Kwon JS, Ferguson S, et al. Simple versus Radical Hysterectomy in Women with Low-Risk Cervical Cancer. N Engl J Med. 2024;390(9):819-829. doi:10.1056/NEJMoa2308900
Exclusion criteria
- Any histology other than squamous cell carcinoma, adenocarcinoma or adenosquamous carcinoma of the cervix uteri
- Tumor size of 4 cm and larger (MRI imaging or clinical examination)
- FIGO stage IB3- IV
- Patients after radiotherapy of the pelvis or abdomen
- Pregnant patients
- Patients with metastatic disease, enlarged pelvic or para-aortic lymph nodes (> 2 cm) or histologically confirmed lymph node metastases Unsuitability for surgery or severe concomitant systemic disease (at the investigator's discretion)
FAQ - Frequently asked questions and our answers
- Is there a maximum period of time between conization and hysterectomy that must not be exceeded?
No. - Can we include patients who have had external conization (and therefore pathology) in the G-LACC?
Yes. - What do we do with women with early cervical cancer who meet the SHAPE criteria?
They can be operated on with a simple hysterectomy in the case of R0 conization. - If a patient fulfills the SHAPE criteria and receives a simple hysterectomy as part of the G-LACC study, do you still need a 5 mm vaginalcuff?
No, the vaginal cuff should not be more than 5 mm. The surgical procedure in this case is similar to a "classic, simple" hysterectomy. - Are patients who meet the SHAPE criteria also randomized to either the abdominal or laparoscopic arm?
Yes, patients who meet the SHAPE criteria are also randomized. This means that it is decided by lot whether a laparotomy or laparoscopy is performed. - Can a hollow adapter cap (=ectocervical manipulator) WITHOUT pin insertion (without transcervical uterine manipulator) be used during the operation for patients after conization and R0 situation post conization?
Yes, this is possible. - How is a patient randomized for the G-LACC study?
Randomization takes place via the eCRF.
Downloads
- Patient information and consent ( Download - DE)
- Patient information and consent (Download - EN)
- Patient information and consent for the collection of biomaterial (Download - DE)
- Patient information and consent for the collection of biomaterial (Download - EN)
- Quality of Life Questionnaire for Cancer Patients (EORTC-QLQ-C30) - Download EN
- Quality of life questionnaire for cancer patients (EORTC-QLQ-C30) - Download EN
- Quality of life questionnaire for cervical cancer patients (EORTC-CX24) - Download EN
- Quality of life questionnaire for cervical cancer patients (EORTC-CX24) - Download EN
- Current quality of life assessment questionnaire (EuroQol EQ-5D-3L) - Download EN
- Current quality of life assessment questionnaire (EuroQol EQ-5D-3L) - Download EN
- Quality of life questionnaire for patients with lymphoedema (LYMQOL) - Download EN
- Quality of Life Questionnaire for Patients with Lymphoedema (LYMQOL) - Download EN
- Sexual Activity and Functionality Questionnaire (SAQ) - Download EN
- Sexual Activity and Functionality Questionnaire (SAQ) - Download EN