More news on the homepage
The humanized liver/mouse model for NAFLD research
Non-alcoholic fatty liver disease (NAFLD) is a rapidly growing global challenge that now affects more than a quarter of the world's population.
The mechanisms by which NAFLD progresses to non-alcoholic steatohepatitis (NASH) and/or further to cirrhosis and even liver cancer are poorly understood and are linked to dietary and genetic risk factors, among others.
In recent years, several genetic polymorphisms have been associated with advanced NAFLD. Of particular note is the 148M variant of the lipase "patatin-like phospholipase domain-containing protein 3" (PNPLA3-I148M), which is associated with advanced NAFLD independent of BMI and/or diabetes status.
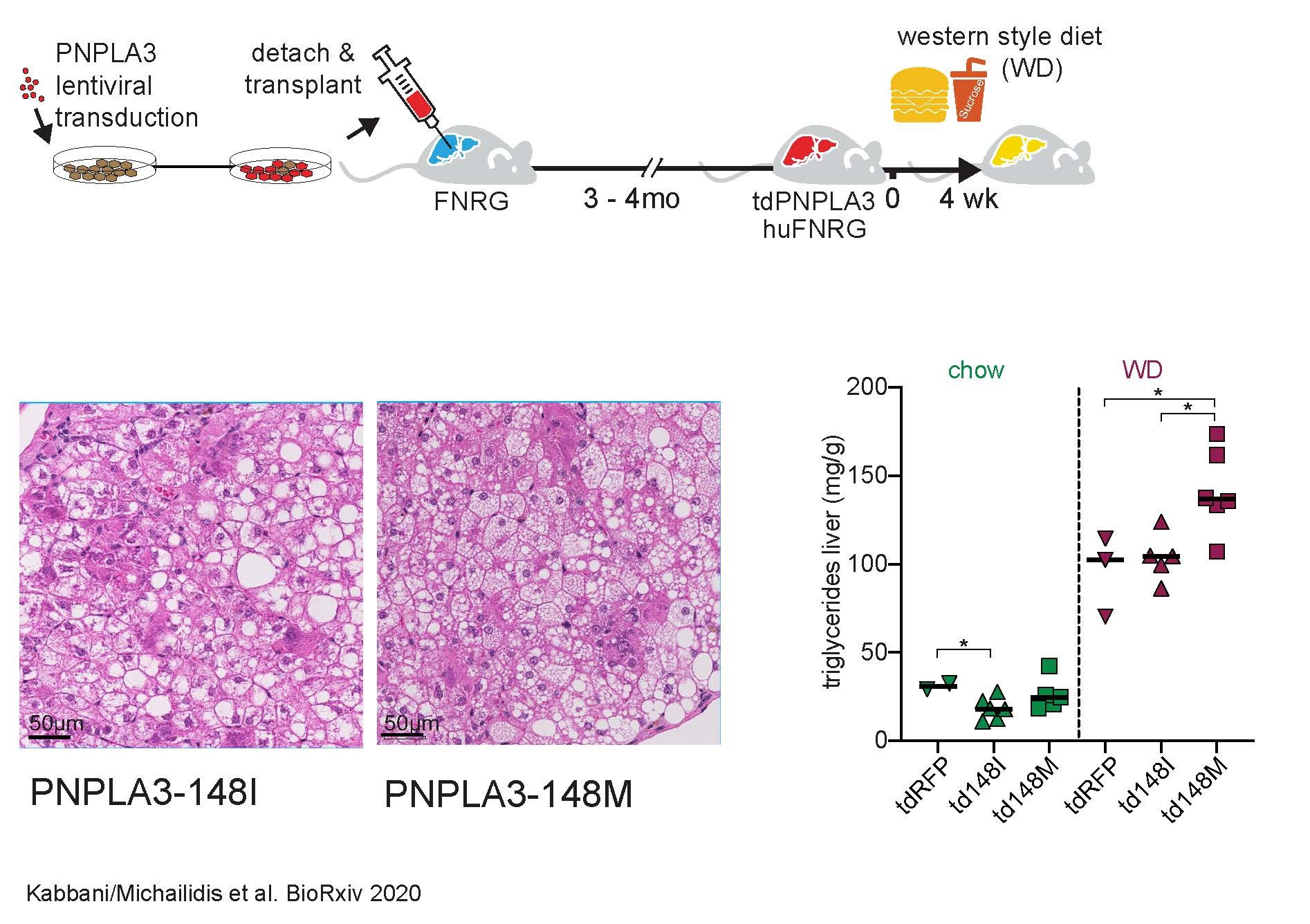
The exact pathomechanisms attributable to the 148M variant in humans remain unclear despite numerous studies. Mouse studies used to date may have hampered the understanding of how the 148M variant causes disease progression: Mouse Pnpla3 is only 68% homologous to human PNPLA3. In addition, human PNPLA3 is mainly expressed in the liver, whereas in mice it is expressed in adipose tissue.
In order to overcome these and other hurdles, we further developed the humanized liver/mouse model for use in NAFLD research as part of a DFG-funded research stay in the laboratory of the 2020 Nobel Prize winner, Prof. Charles Rice, at Rockefeller University in New York.
Here, human hepatocytes are injected into immunocompromised mice and replace a large proportion of the mouse liver cells so that the mouse liver is "humanized". By humanizing mice with hepatocytes of the 148M variant (either as carriers of the variant or by means of forced lentiviral overexpression) following a four-week hyperacaloric Western-style diet (a type of fast food diet), we were able to show that the 148M variant leads to particularly aggressive NAFLD, including the development of microvesicular steatosis. Of particular interest, however, was the observation that steatoheapatitis occurred in the humanized mice despite the absence of T and B cells, including an increase in liver enzymes and the ballooning of hepatocytes. This underlines even more the central role of hepatocytes in the development of steatohepatitis - independent of an adaptive immune system.
Next, we would like to establish the humanized liver/mouse model for NAFLD research at the MHH in order to further investigate the influence of different hypercaloric diets in the context of genetic risk factors. Other genetic risk factors for NAFLD that could not be investigated in mouse models so far, e.g. because no murine orthologue has been described so far (e.g. HSD17B13), can also be investigated in the model. In the long term, therapeutic interventions in the humanized liver/mouse model are also conceivable, in particular the use of innovative gene therapies on human hepatocytes in vivo.
For further information please contact: Dr. Mohammad Kabbani, kabbani.mohammad@mh-hannover.de
