Profile of the Institute of Cell Therapeutics
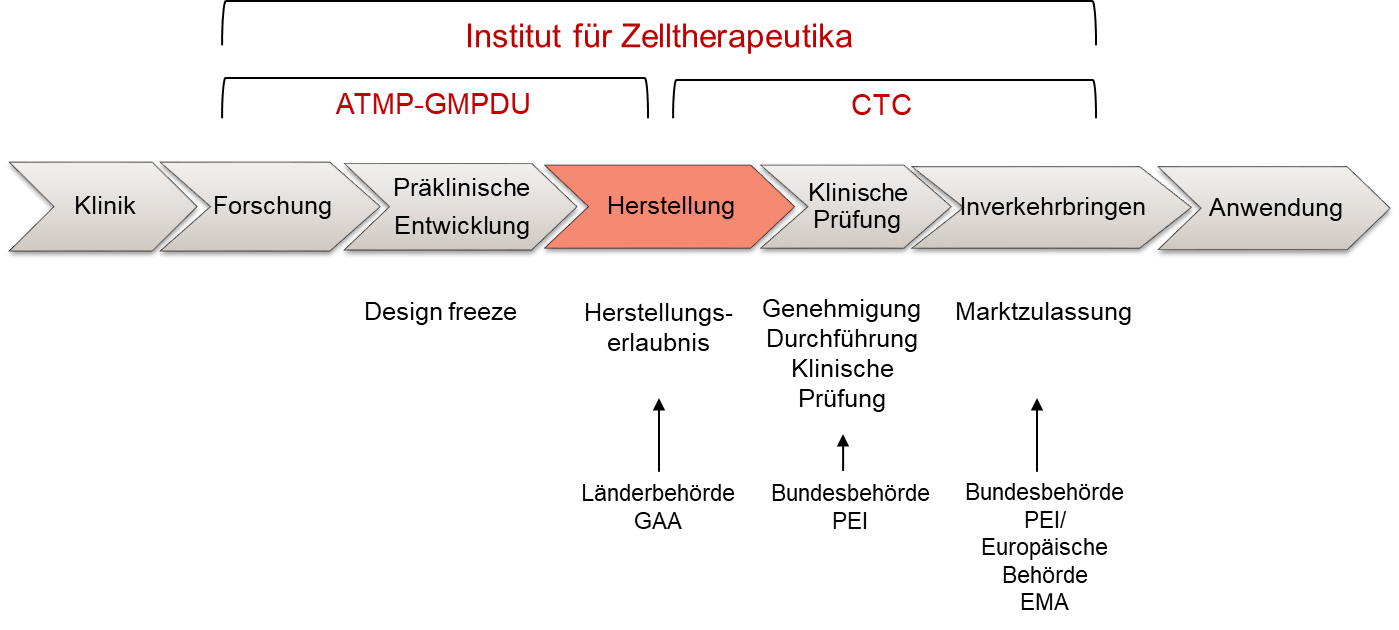
The Institute of Cell Therapeutics comprises two Departments, the GMP Development Unit(ATMP-GMPDU) and the Cellular Therapy Center(CTC) as the manufacturing and testing facility of the MHH.
The focus of the ATMP-GMPDU is onthe development and implementation of processes for the GMP-compliant production of cell therapeutics. This includes various hematopoietic and stromal cell types, e.g. T cells, CAR T and CAR NK cells, TRUCKs, induced DCs, Tregs, MSCs and iPS cells. The task of our central Facilities or Institutions is to advise and support research groups in the translational implementation of their projects.
The CTC is responsible for the production of cellular drugs for the treatment of serious diseases, especially of the hematopoietic system. Production is carried out in accordance with national and European guidelines, technical regulations and the current state of medical and pharmaceutical science and technology. The CTC has all the necessary skills, equipment and staff (e.g. qualified persons, quality control and manufacturing managers) etc. as well as the necessary Facilities or Institutions (Class A/B clean rooms in accordance with EU GMP guidelines).
The close link between the ATMP-GMPDU development department and the CTC manufacturing site enables a step-by-step translation from the scientific idea to the production of a cellular drug.