MHH scientists are investigating how pro-inflammatory cytokines intervene in the metabolism of muscles and actively remodel them.
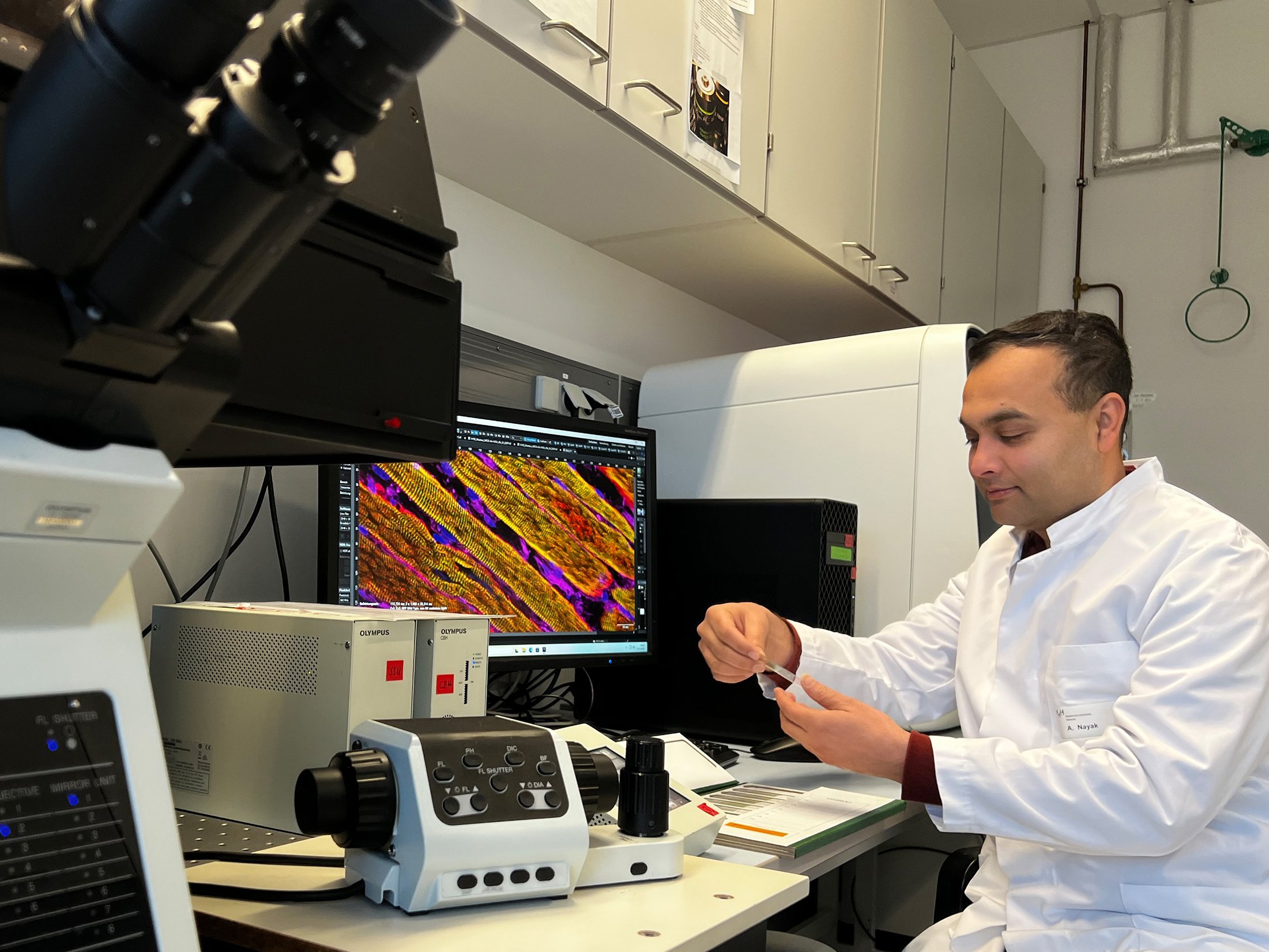
Dr Arnab Nayak, private lecturer, investigates the effect of pro-inflammatory messengers on muscle wasting in cancer. Copyright: Tim Holler/MHH
Cancer patients often lose an excessive amount of weight. This affects up to 80 percent of patients and is mainly due to the loss of muscle mass and fatty tissue. This cancer-induced cachexia (CIC) is triggered by cancer cytokines, i.e. inflammation-promoting messenger substances that the tumor cells themselves emit. It is not uncommon for the heart muscle to be affected, which further weakens the patient. Depending on the type of cancer, CIC is responsible for 20 to 50 percent of all cancer-related deaths worldwide. There is no curative therapy. In order to find an effective treatment approach, a research team led by associate professor (PD) Dr. Arnab Nayak, a scientist at the Institute of Molecular and Cell Physiology at Hannover Medical School (MHH), has been investigating the previously unknown molecular mechanisms associated with CIC. With his "Chromatin and SUMO Physiology" working group, the molecular biologist has shown that the cancer cytokines intervene directly in the metabolism of muscle cells and actively remodel them. They also ensure that the muscle can release less calcium, which impairs muscle contraction, i.e. the active contraction of the muscle. The results have been published in the "Journal of Cachexia, Sarcopenia and Muscle".
Loss of the ability to contract
The researchers investigated the effect of CIC on the skeletal and heart muscle cells of mice and rats in cell culture. They tested the contractile properties of the muscle cells after electrical stimulation and measured the release of calcium within the muscle cells. They also used a high-resolution microscope to monitor how CIC affects the organization of the sarcomeres, the smallest functional units of the muscle. They also analyzed the signal transmissions in the cells that regulate which muscle-specific genes are switched on or off. "We observed a drastic loss of contraction of striated muscle cells in CIC, which was primarily due to acutely disorganized sarcomere structures and an impaired calcium transport process," states PD Dr. Nayak.
However, the proinflammatory cytokines not only reduce the muscle's ability to contract. They also destroy the muscle cells themselves. On the one hand, they activate an enzyme that marks muscle proteins for degradation. The system is supposed to remove faulty proteins from the cell. In this case, however, the degradation system ensures that functioning muscle proteins are destroyed. In addition, the cytokines influence a central signaling pathway within the muscle cells that regulates their growth, division, metabolism and survival.
SUMO signaling pathway as a therapeutic approach
Current therapies tend to focus on alleviating the symptoms. For example, dietary supplements such as polyunsaturated omega-3 fatty acids in combination with vitamin D3 and endurance and strength exercises are used in an attempt to stop muscle atrophy. Cardiac medication such as ACE inhibitors or beta-blockers should also help to at least reduce muscle loss. PD Dr. Nayak relies on molecular biological methods, or more precisely, the so-called SUMO signaling pathway. In this mechanism, the protein SUMO (small ubiqutin-like modifier) is bound to other proteins in order to change their function. The SUMO signaling pathway plays an important role in muscle breakdown. The SUMO-specific enzymes SENP3 and SENP7 regulate epigenetic processes in the muscle-specific genes. These control gene activity without changing the DNA itself.
In cachexia, the enzymes are degraded and, as a result, their muscle-specific target genes are downregulated. As a result, the sarcomeres, the smallest functional units of the muscle, can no longer form as intended and the muscles lose their ability to develop strength. "In our study, we upregulated SENP3 and SENP7 and observed a decrease in muscle degradation," says the molecular biologist. However, whether this approach works beyond cell culture still needs to be investigated further. PD Dr. Nayak and his team therefore want to test the muscle-saving effect in a mouse model next.
Text: Kirsten Pötzke
SERVICE:
The original paper "Calcium Handling Machinery and Sarcomere Assembly are Impaired Through Multipronged Mechanisms in Cancer Cytokine-Induced Cachexia" can be found here.