Research report 2012
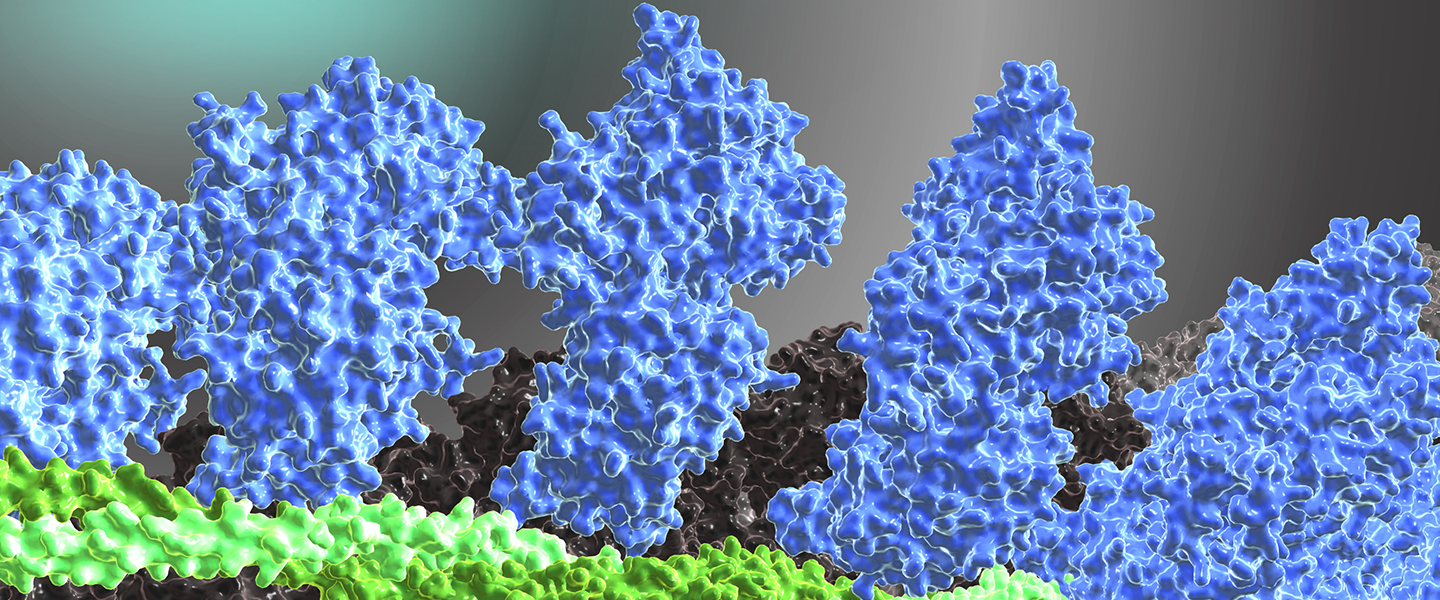
The cover image of the 2012 research report shows a computer-chemically generated structural model of the actomyosin-tropomyosin complex (ATM complex) of human non-muscular myosin-2B (blue), g-actin (gray) and tropomyosin (green). The model is based on the recently experimentally solved structure of an ATM complex consisting of myosin-1, tropomyosin and α-actin(Behrmann et al., Cell, 150, 327-338, 2012).
The models provide new insights into the regulation of actomyosin motor function by tropomyosin, the mechanism of chemomechanical coupling in the myosin motor domain and form the basis for the development of therapeutic approaches for the treatment of diseases caused by mutations of the contractile proteins.
In the accompanying animation, the reactive thiol region (SH1/SH2) in the myosin motor domain, which is a focal point of allosteric Communications, is also highlighted. Through the combined use of structural, computational chemistry and biophysical methods, the mechanism leading to the disruption of force development and ATP hydrolysis by the point mutations G680V and G680A could be elucidated, whereby an uncoupling of crucial structural elements in this region takes place(Preller et al., J. Biol. Chem., 286, 35051-35060, 2011).
Research report 2012
Research Report 2012
Editor:
The Dean of Research at Hannover Medical School
Prof. Dr. med. Christopher H. Baum
Editing and contact person:
Office of the Dean of Research at Hannover Medical School
Petra Linke
Phone: 0511/ 532- 6023
Fax: 0511/ 532- 6024
E-mail: linke.petra@mh-hannover.de
Design and typesetting:
Digital Media, Hannover Medical School
Joachim Barke
Phone: 0511/ 532- 2963
Production:
Digital Media, Hannover Medical School
Telephone: 05 11/ 532- 2963
Wewould like to thank the staff of the library of the Hannover Medical School, Usage Department: Ms. Ingeborg Heering, for her support with the bibliographic information.
Wewould like to thank the staff of the Center for Information Management (ZIMt) under the direction of Mr. Dirk May for their support in the implementation of the ICT-supported data collection and the preparation of the printing process.
Alldata(including the links contained) in the research report are based on the information provided by the respective Facilities or Institutions. The entry is made without guarantee.
Themasculine form of all gender-specific descriptions applies accordingly to the feminine form.