Research report 2014
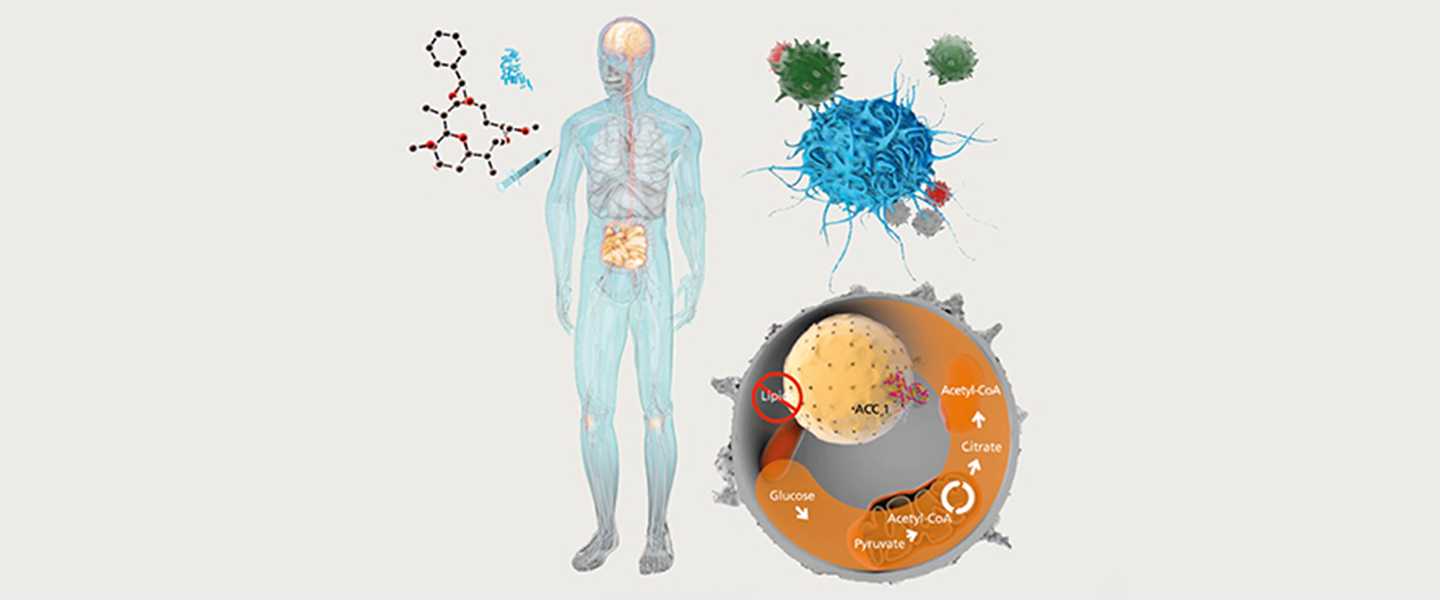
Normally, T helper cells play an important role in the defense against bacteria, viruses, fungi and parasites. However, the messenger substances (so-called cytokines) released by T cells can also have a strong pro-inflammatory effect. If the control of the T-cell response fails, this can lead to chronic inflammation, which can even be directed specifically against the body's own tissue, as in the case of autoimmune diseases.
Changes in intracellular metabolism have a significant influence on the differentiation and function of T cells.
A study from the Institute of Infection Immunology at Twincore (Berod et al. Nat Med. 2014 20:1327-33) was able to show that the development of inflammatory CD4+ T helper cells depends on the activation of intracellular de novo fatty acid synthesis. Blockade of a key enzyme of fatty acid synthesis, acetyl-CoA carboxylase (ACC)1, by the myxobacterial agent soraphen A not only very effectively inhibits the development of murine and human inflammatory T cells, but also simultaneously favors the development of anti-inflammatory regulatory T cells. These results point to a completely new way of modulating the balance between inflammatory and regulatory T cells in autoimmune or infection-associated processes in the body by directly interfering with intracellular lipid metabolism.
Research report 2014
Research Report 2014
Publisher:
President of the MHH
Prof. Dr. med. Christopher H. Baum
Dean of Research of the MHH
Prof. Dr. Dr. phil. Denise Hilfiker-Kleiner, PhD
Processing and contact person:
Office of the Dean of Research at Hannover Medical School
Petra Linke
Phone: 0511/ 532- 6023
Fax: 0511/ 532- 6024
E-mail: linke.petra@mh-hannover.de
Design and typesetting:
Digital Media, Hannover Medical School
Joachim Barke
Phone: 0511/ 532- 2963
Production:
Digital Media, Hannover Medical School
Telephone: 05 11/ 532- 2963
Wewould like to thank the staff of the library of the Hannover Medical School, Usage Department: Ms. Ingeborg Heering, for her support with the bibliographic information.
Wewould like to thank the staff of the Center for Information Management (ZIMt) under the direction of Mr. Dirk May for their support in the implementation of the ICT-supported data collection and the preparation of the printing process.
Alldata(including the links contained) in the research report are based on the information provided by the respective Facilities or Institutions. The entry is made without guarantee.
Themasculine form of all gender-specific descriptions applies accordingly to the feminine form.
Cover picture:
The cover image schematically shows how the development of inflammatory T cells can be influenced by targeted intervention in cellular fatty acid metabolism. Normally, T helper cells play an important role in the defense against bacteria, viruses, fungi and parasites. However, the messenger substances (so-called cytokines) released by T cells can also have a strong pro-inflammatory effect. If the control of the T-cell response fails, this can lead to chronic inflammation, which can even be directed specifically against the body's own tissue, as in the case of autoimmune diseases.
Changes in intracellular metabolism have a significant influence on the differentiation and function of T cells. A study from the Institute of Infection Immunology at Twincore(Berod et al. Nat Med. 2014 20:1327-33) was able to show that the development of inflammatory CD4+ T helper cells depends on the activation of intracellular de novo fatty acid synthesis. The blockade of a key enzyme of fatty acid synthesis, acetyl-CoA carboxylase (ACC)1 , by the myxobacterial agent soraphen A not only very effectively inhibits the development of murine and human inflammatory T cells, but also simultaneously favors the development of anti-inflammatory regulatory T cells. These results point to a completely new way of modulating the balance between inflammatory and regulatory T cells in autoimmune or infection-associated processes in the body by directly interfering with intracellular lipid metabolism. The image was provided by Prof. Dr. Tim Sparwasser, Director of the Institute of Infection Immunology.