Background
The number of patients examined in clinical studies up to the market approval of a drug is limited and may only be transferable to everyday clinical practice to a limited extent due to the artificial setting of such admission studies. Post-marketing surveillance of a drug is therefore of particular importance.
According to the definition of the World Health Organization (WHO), pharmacovigilance comprises the analysis and prevention of drug risks and the development of activities to detect, assess, understand and prevent adverse drug reactions (ADRs). Detection and analysis of serious adverse drug reactions and their prevention are thus also part of an "error culture" in Clinical Departments.
For an enlarged view of the graphic on the right, please click here.
Overarching goals
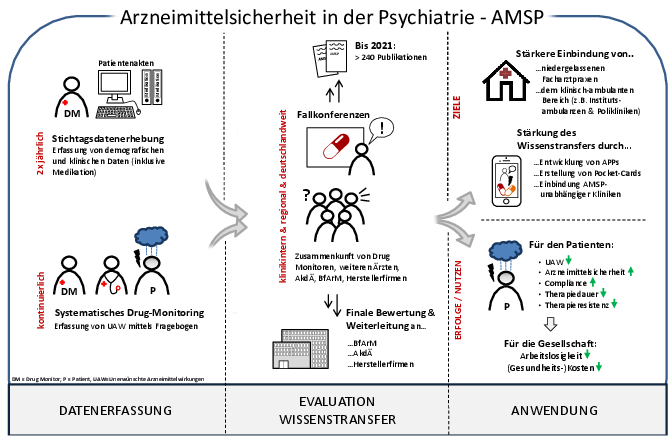
The overall aim of the "Drug Safety in Psychiatry" project is to improve drug safety in the treatment of psychiatric patients. In a total of over 50 project clinics (Germany, Austria, Switzerland), "adverse drug reactions" (ADRs) involving psychotropic drugs are continuously recorded, currently mainly in the inpatient sector (non-interventional).
The aims are to detect, analyze and assess the causality of serious ADRs. In this way, incidences of ADRs and specific risk profiles of substance groups or individual substances can be determined and compared with each other.
The focus is on analyzing the significance of
- drug interactions,
- risk combinations,
- polypharmacy,
- relevant patient-related variables (age, gender) as well as
- the relevance of pharmacogenetics (polymorphisms)
in psychopharmacological treatment.
The project is also designed as a system that has a signal and alarm function in the field of psychopharmacotherapy for the occurrence of clinically significant or novel ADRs. Drug monitoring in psychiatry is a component of quality assurance in treatment and part of clinical risk management. Drug safety should be anchored as part of a "treatment culture" within the framework of a comprehensive patient protection concept.
Current projects
The current severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic has changed almost all areas of medicine and public life - including psychopharmacotherapy. A recent publication in "Nervenarzt" highlights clinically relevant aspects that require increased attention due to the increased risk of infection. These include recommendations regarding the readjustment of patients to clozapine, but also possible interactions such as an increased risk of thromboembolic events, changes in drug metabolism via the enzymes of the cytochrome P450 system and respiratory depressive effects.
Resulting publication:
Seifert J, Heck J, Eckermann G, Singer M, Bleich S, Grohmann R, Toto S. Psychopharmacotherapy in Zeiten der COVID-19-Pandemie [Psychopharmacotherapy during the COVID-19 pandemic]. Neurologist. 2020 Jul;91(7):604-610. PMID: 32488413; PMCID: PMC7265158. DOI
Drug-induced liver cell damage can have extremely serious consequences for patients. It is of great clinical relevance to understand under which active substances these can occur, as psychiatric patients often also have other risk factors (e.g. substance use, unhealthy diet). In a study on drug-induced liver cell damage, the risk of individual antipsychotics is analyzed.
Resulting publication:
Druschky K, Toto S, Bleich S, Baumgärtner J, Engel RR, Grohmann R, Maier HB, Neyazi A, Rudolph YJ, Rüther E, Schwörer H, Seifert J, Stübner S, Degner D. Severe drug-induced liver injury in patients under treatment with antipsychotic drugs: data from the AMSP study. World J Biol Psychiatry. 2020 Sep 7:1-29. PMID: 32892689. DOI
Depression is one of the most common psychiatric illnesses. One of the most important therapeutic approaches is psychopharmacotherapy, which includes not only the administration of antidepressants. At the MHH, data is currently being analyzed under various questions in order to achieve a differentiated understanding of psychopharmacotherapy in depressive patients. One of the questions is to what extent the choice of active substances differs between men and women. Furthermore, changes in the psychopharmacotherapy of major depression over a period of 17 years are examined.
Scientific collaboration
More than 50 psychiatric clinics from Germany, Austria and Switzerland are currently participating in the AMSP project. The Clinical Department of Psychiatry and Psychotherapy at the Medical University of Munich is the headquarters of the AMSP and part of the center of the "Regional Group North" in close cooperation with the Ludwig-Maximilians-Universität München. The project is in close cooperation with state institutions at national and international level (for example with the Federal Institute for Drugs and Medical Devices, BfArM; the "Drug Commission of the German Medical Association" (AkdÄ)) and is a member of the "European Network of Centers for Pharmacoepidemiology and Pharmacovigilance" ENCePP under the direction of the "European Medicines Agency" EMA.
Special cooperation partners:
- Clinical Department of Psychiatry and Psychotherapy, LMU Munich (Prof. Dr. R. Grohmann, Prof. E. Rüther)
- Clinical Department of Psychiatry and Psychotherapy, UMG Göttingen (Prof. D. Degner)
- Institute of Clinical Pharmacology, MHH Hannover (Prof. D. O. Stichtenoth, Dr. J. Heck)
Research group members
Head of research group
Prof. Dr. med. Stefan Bleich
Medical Director, AMSP Chairman
Telephone: 0511 / 532-6748
PD Dr. med. Sermin Toto
Managing Senior Physician, Specialist in Psychiatry and Psychotherapy
Phone: 0511 / 532-2403
Office
Marzena Schaefer
Phone: +49 511 532 5565
Fax: +49 511 532 18573
