Genth working group

Head of the working group:
Prof. Dr. rer. nat. Harald Genth
Building J06, Level 03, Room 2561
Members of the working group:
Ilona Schelle
Finn Schäfer (Bachelor Biology)
Research
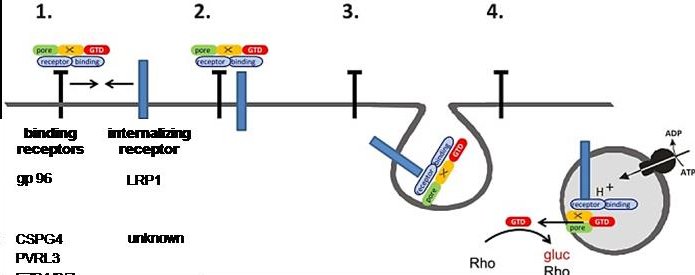
Investigation into the cellular uptake of C. difficile toxins
Toxigenic strains of C. difficile are the main cause of antibiotic-associated diarrhea and its severe form, pseudomembranous colitis. C. difficiole produces three enterotoxins, toxin A (tcdA), toxin B (TcdB) and the C. difficile toxin (CDT). TcdA (307 kDa) and TcdB (270 kDa) are large, single-chain protein toxins that insert themselves into their target cells by means of receptor-mediated endocytosis. In the cell, TcdA and TcdB mono-O-glucosylate (thus inactivate) low-molecular GTP-binding proteins of the Rho and Ras families, as a result of which the barrier function of the colonic epithelium and colon renewal are inhibited. In the last 5 years, the identification of toxin receptors has gained momentum.
The Genth group has recently developed a new 2-receptor model of toxin uptake, which distinguishes between internalizing and non-internalizing receptors in toxin uptake. The internalization of toxin receptors is investigated by reversibly biotinylating the receptors at the cell surface and inducing the internalization of biotinylated receptors by increasing the temperature (cooperation with Dr. Robert Lindner, MHH Anatomy). Other important techniques allow the determination of cell surface binding of toxins and toxin-receptor binding. In addition, the endocytosis mechanism by which the toxins reach the cytosol of the target cell will be identified. This work allows the identification of pharmacological target structures for the development of antitoxins.
Structure-function analysis of cytotoxins from Chlamydia spp.
The Gram-negative, intracellular bacterium Chlamydia trachomatis causes acute and chronic infections of the urogenital tract, which can lead to infertility and ectopic pregnancy. The only partially characterized cytotoxin CT166 of Chlamydia trachomatis serovar D shows striking similarities to C. difficile toxins. We have identified cytotoxins related to CT166 in various Chlamydia spp. Therefore, the Genth research group, in cooperation with Prof. Andreas Klos (MHH Medical Microbiology), is investigating the biological effects of this toxin family, which has hardly been studied to date.