Brain tumors
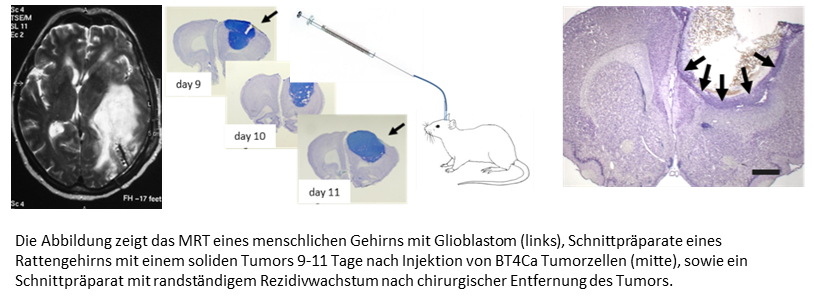
Despite considerable progress in neuro-oncological research, the treatment of brain tumors remains problematic. This applies in particular to glioblastoma, the most prognostically unfavorable malignant brain tumor in adults. Despite a combination therapy of microsurgical removal (resection) and concomitant radiochemotherapy, the average survival time is still only one to two years. Due to the infiltrative growth pattern, the recurrence rate after resection is up to 90% after one year.
One focus of the Clinical Department's experimental laboratory is neuro-oncological research with tumor cells derived from human brain tumors and with tumor cell lines. As an animal model, we use the BT4Ca glioblastoma cell line, which grows into a solid tumor with infiltrative growth within a few days after stereotactic injection into frontal brain regions of rats. Shortly after microsurgical resection, only marginal tumor cells are detectable, from which a solid tumor develops again after about two weeks. This rat model is suitable for testing the local efficacy of drugs, either after microinjection or after administration into the resection cavity. In a similar setup, we also want to test the effect of a high-frequency alternating electric field (TTF) on tumor growth. The effectiveness of such a field on the growth of tumor cells in cell culture has already been demonstrated.
Helgers SOA, Talbot SR, Riedesel AK, Wassermann L, Wu Z, Krauss JK, Häger C, Bleich A, Schwabe K. Body weight algorithm predicts human endpoint in an intracranial rat glioma model. Sci Rep. 2020 Jun 2;10(1):9020. DOI: 10.1038/s41598-020-65783-7
Berkelmann L, Bader A, Meshksar S, Dierks A, Hatipoglu Majernik G, Krauss JK, Schwabe K, Manteuffel D, Ngezahayo A. Tumor-treating fields (TTFields): Investigations on the mechanism of action by electromagnetic exposure of cells in telophase/cytokinesis. Sci Rep. 2019 May 14;9(1):7362. DOI: 10.1038/s41598-019-43621-9
Wu Z, Nakamura M, Krauss JK, Schwabe K, John N. Intracranial rat glioma model for tumor resection and local treatment. J Neurosci Methods. 2018 Apr 1;299:1-7. DOI: 10.1016/j.jneumeth.2018.02.002
Borrmann N, Friedrich S, Schwabe K, Hedrich HJ, Krauss JK, Knapp WH, Nakamura M, Meyer GJ, Walte A. Systemic treatment with 4-211Atphenylalanine enhances survival of rats with intracranial glioblastoma. Nuclear Medicine. 2013 Dec 13;52(6):212-21. DOI: 10.3413/Nukmed-0580-13-05
Friedrich S, Schwabe K, Grote M, Krauss JK, Nakamura M. Effect of systemic celecoxib on human meningioma after intracranial transplantation into nude mice. Acta Neurochir (Vienna). 2013 Jan;155(1):173-82. DOI: 10.1007/s00701-012-1534-7
Friedrich S, Schwabe K, Klein R, Krusche CA, Krauss JK, Nakamura M. Comparative morphological and immunohistochemical study of human meningioma after intracranial transplantation into nude mice. J Neurosci Methods. 2012 Mar 30;205(1):1-9. DOI: 10.1016/j.jneumeth.2011.12.009
Hong B, Krusche CA, Schwabe K, Friedrich S, Klein R, Krauss JK, Nakamura M. Cyclooxygenase-2 supports tumor proliferation in vestibular schwannomas. Neurosurgery. 2011 Apr;68(4):1112-7. DOI: 10.1227/NEU.0b013e318208f5c7
Collaborations
Prof. Dr. Christian Hartmann (Institute of Neuropathology, MHH)
Prof. Dr. Thomas Skripuletz (Clinical Department of Neurology, MHH)
Prof. Dr. Dirk Manteuffel (Institute for High Frequency Technology, LUH)
Prof. Dr. Anaclet Ngezahayo (Institute of Biophysics, LUH)
Prof. Dr. Dr. Hannelore Ehrenreich (Max Planck Institute for Experimental Medicine, Göttingen)
Funding
DFG Research Unit 2591 - SCHW1176/7-1
Neurosurgical Research Foundation