AG Lung Research
Welcome

The Lung Research Group is an interdisciplinary research group at the Institute of Pathology of the MHH and at the BREATH site of the German Center for Lung Research(DZL). In addition to interstitial and vascular lung diseases, the research focus of the working group also includes the pathophysiology of COVID-19 disease and its late effects as well as the pathophysiology of changes after lung transplantation. The Lung Research Group is characterized by a national and international network with numerous research partners from all over the world. For collaboration requests please contact us.
Yours sincerely,
Lavinia Neubert and Jan C. Kamp
Research focus of the AG Lung Research
Pulmonary hypertension describes a pathological increase in pressure in the small circulation. According to the current definition, a mean pulmonary arterial pressure (PAP) of 20 mmHg is the threshold value. The causes of pulmonary hypertension are varied and the pathophysiology complex. In addition to primary diseases of the pulmonary arterial vascular wall, congenital heart defects, connective tissue diseases, left heart diseases, chronic lung diseases, chronic thromboembolic diseases and many other changes can result in pulmonary hypertension.
Hemodynamically, a distinction can be made between pre-capillary forms, which are characterized by an increase in pulmonary vascular resistance (PVR) and a low pulmonary arterial occlusion pressure (PAWP), and post-capillary forms caused by left heart disease. Untreated, the median 5-year survival is about 30%. A curative therapy is not yet available, leaving only lung transplantation as a last resort in the final stage of the disease.
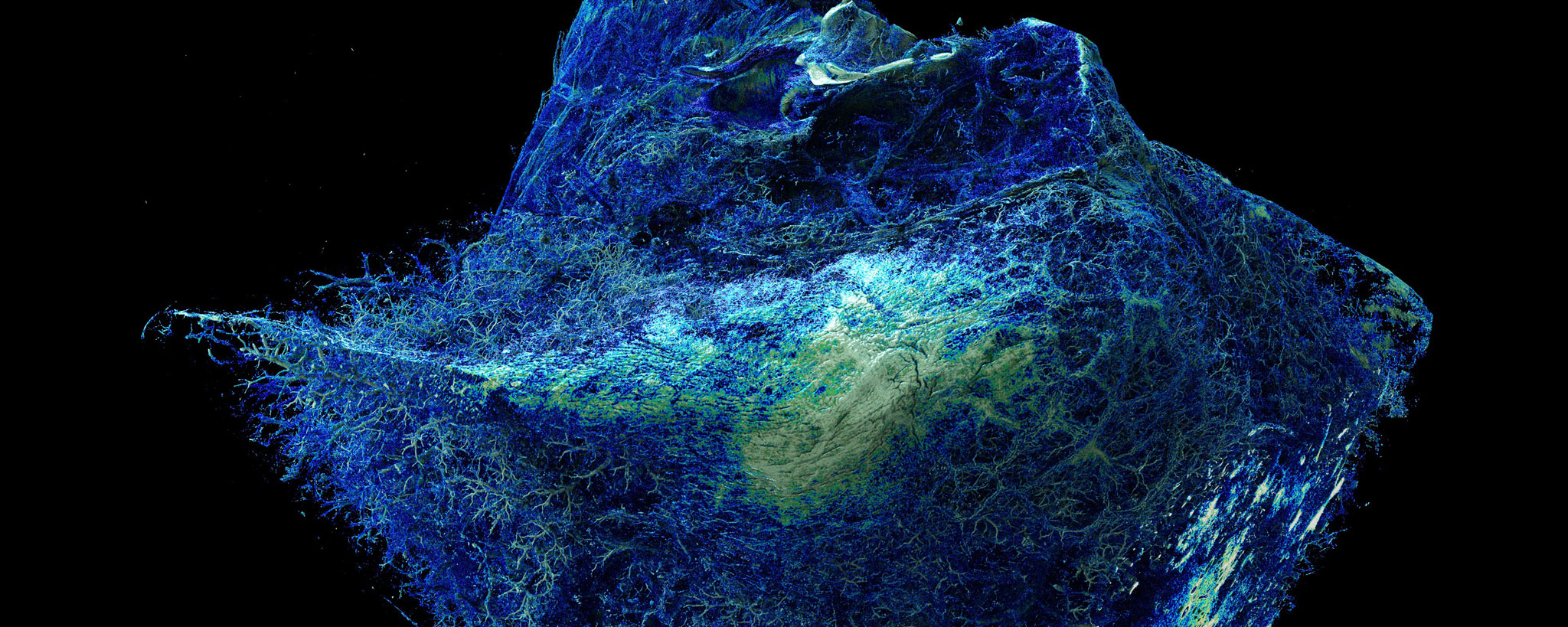
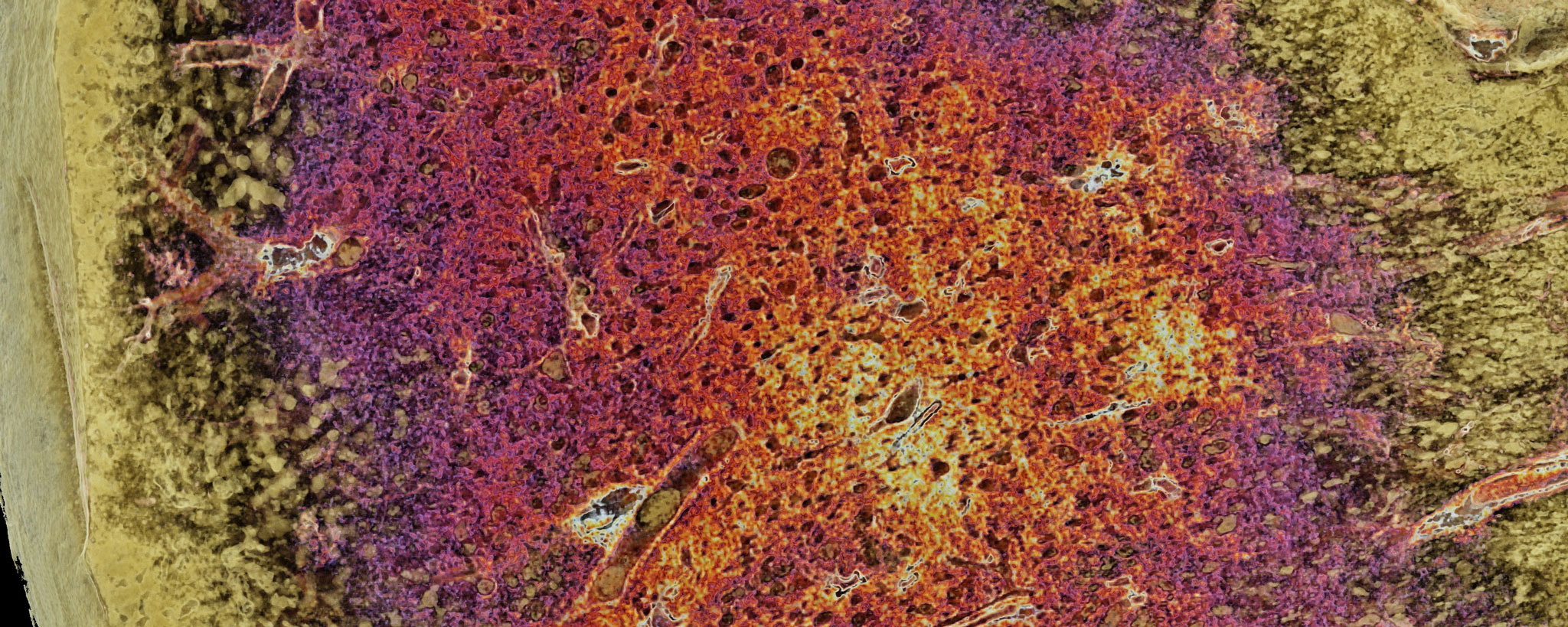
Histomorphological signs of advanced PAH are so-called plexiform and concentric lesions, which are an expression of complex vascular remodeling. Whether these lesions are merely the result of increased intravascular pressure or actively contribute to the progression of the disease is currently the subject of research. It is generally accepted that plexiform lesions are misdirected neovascularizations resulting from an imbalance between endothelial apoptosis and necrosis, followed by circumscribed endothelial proliferation.
The limited availability of fresh PAH lung tissue poses a challenge for translational studies of this disease. Opportunities for in vitro analysis of underlying molecular mechanisms exist e.g. in the Sugen hypoxia rat model or the monocrotaline mouse model as well as in the use of tissue cultures from precision cut lung slices (PCLS).
Selected publications
Kamp JC, Neubert L, Schupp JC, Braubach P, Wrede C, Laenger F, Salditt T, Reichmann J, Welte T, Ruhparwar A, Ius F, Schwerk N, Bergmann AK, von Hardenberg S, Griese M, Rapp C, Olsson KM, Fuge J, Park DH, Hoeper MM, Jonigk DD, Knudsen L, Kuehnel MP. Multilamellated Basement Membranes in the Capillary Network of Alveolar Capillary Dysplasia. Am J Pathol. 2024 Feb;194(2):180-194. doi: 10.1016/j.ajpath.2023.10.012.
Olsson KM, Corte TJ, Kamp JC, Montani D, Nathan SD, Neubert L, Price LC, Kiely DG. Pulmonary hypertension associated with lung disease: new insights into pathomechanisms, diagnosis, and management. Lancet Respir Med. 2023 Sep;11(9):820-835. doi: 10.1016/S2213-2600(23)00259-X.
Kamp JC, Neubert L, Ackermann M, Stark H, Plucinski E, Shah HR, Janciauskiene S, Bergmann AK, Schmidt G, Welte T, Haverich A, Werlein C, Braubach P, Laenger F, Schwerk N, Olsson KM, Fuge J, Park DH, Schupp JC, Hoeper MM, Kuehnel MP, Jonigk DD. A Morphomolecular Approach to Alveolar Capillary Dysplasia. Am J Pathol. 2022 Aug;192(8):1110-1121. doi: 10.1016/j.ajpath.2022.05.004.
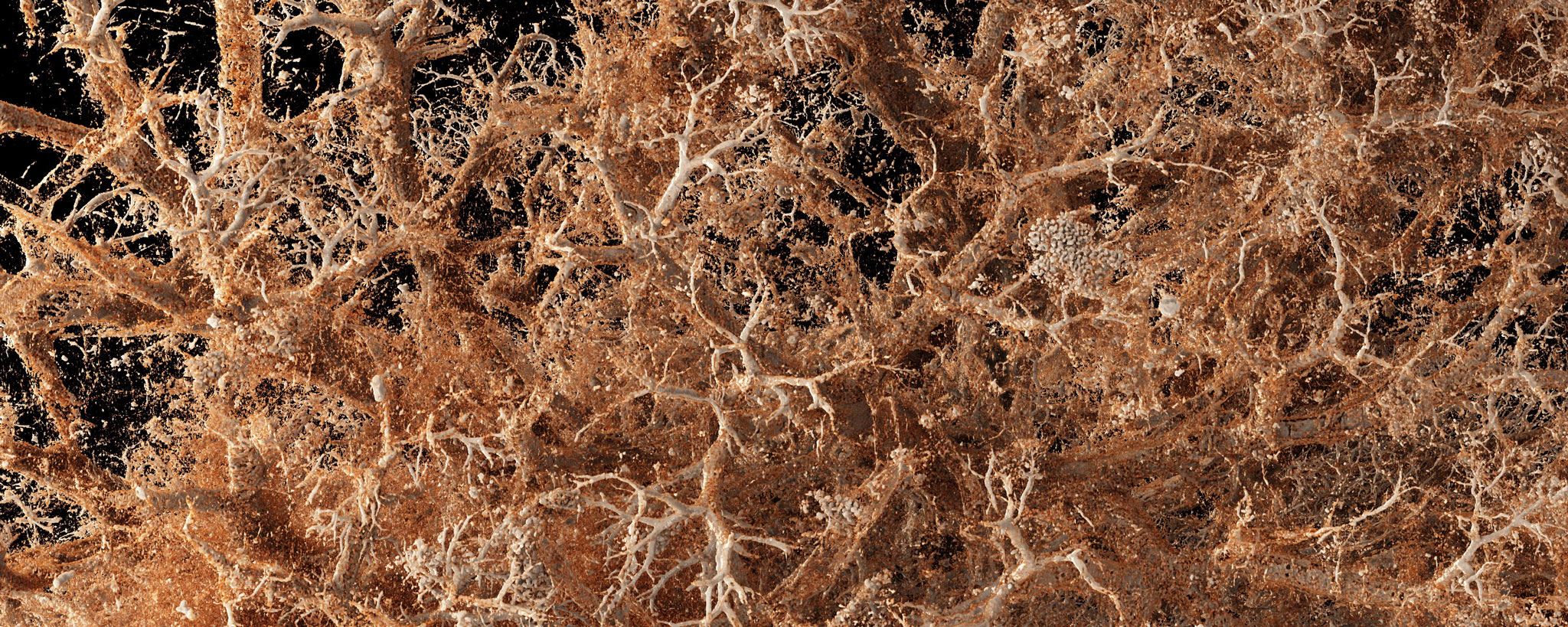
Neubert L, Borchert P, Stark H, Hoefer A, Vogel-Claussen J, Warnecke G, Eubel H, Kuenzler P, Kreipe HH, Hoeper MM, Kuehnel M, Jonigk D. Molecular Profiling of Vascular Remodeling in Chronic Pulmonary Disease. Am J Pathol. 2020 Jul;190(7):1382-1396. doi: 10.1016/j.ajpath.2020.03.008.
Neubert L, Borchert P, Shin HO, Linz F, Wagner WL, Warnecke G, Laenger F, Haverich A, Stark H, Hoeper MM, Kuehnel M, Ackermann M, Jonigk D. Comprehensive three-dimensional morphology of neoangiogenesis in pulmonary veno-occlusive disease and pulmonary capillary hemangiomatosis. J Pathol Clin Res. 2019 Apr;5(2):108-114. doi: 10.1002/cjp2.125.
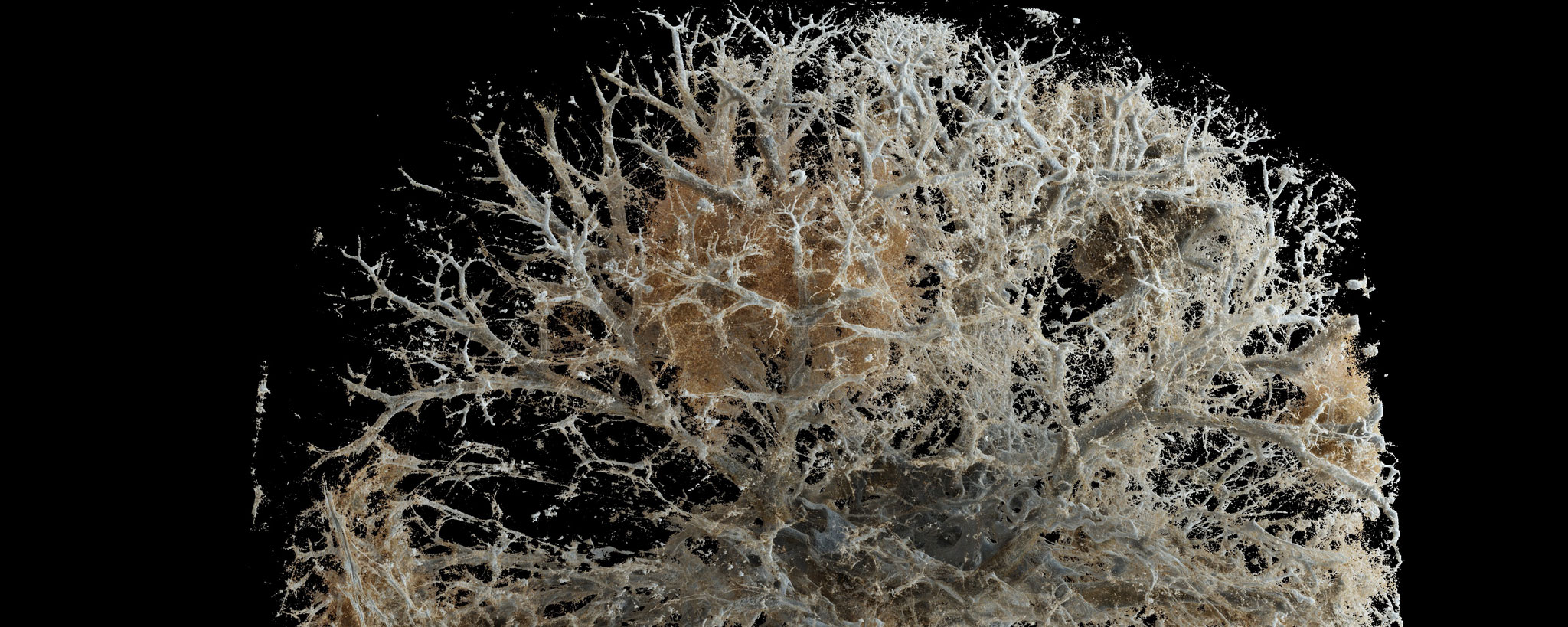
Usual interstitial pneumonia (UIP) is the typical histological manifestation of idiopathic pulmonary fibrosis (IPF). UIP is the most common of the idiopathic interstitial pneumonias and is a progressive, usually fatal disease with very limited treatment options. It is therefore important to differentiate UIP from other interstitial pneumonias on the one hand and from other interstitial lung diseases with a better response to therapy and a better prognosis on the other.
Histopathologically, UIP is characterized by discontinuous, patchy and temporally heterogeneous interstitial fibrosis driven by aggregates of myofibroblasts, so-called (myo-) fibroblastic foci, with secondary changes and proliferation of smooth muscle. Typically, (myo-) fibroblastic foci appear as conglomerates localized mainly in the pulmonary interstitium below the alveolar epithelium, with the long axis of the spindle-shaped myofibroblasts running parallel to the epithelial lining. Although fibroblastic foci are not specific for UIP, their presence in the clinical and pathologic context greatly supports the diagnosis.
Selected publications
Kamp JC, Neubert L, Stark H, Hinrichs JB, Boekhoff C, Seidel AD, Ius F, Haverich A, Gottlieb J, Welte T, Braubach P, Laenger F, Hoeper MM, Kuehnel MP, Jonigk DD. Comparative Analysis of Gene Expression in Fibroblastic Foci in Patients with Idiopathic Pulmonary Fibrosis and Pulmonary Sarcoidosis. Cells. 2022 Feb 14;11(4):664. doi: 10.3390/cells11040664.
Preuß EB, Schubert S, Werlein C, Stark H, Braubach P, Höfer A, Plucinski EKJ, Shah HR, Geffers R, Sewald K, Braun A, Jonigk DD, Kühnel MP. The Challenge of Long-Term Cultivation of Human Precision-Cut Lung Slices. Am J Pathol. 2022 Feb;192(2):239-253. doi: 10.1016/j.ajpath.2021.10.020.
Braubach P, Werlein C, Verleden SE, Maerzke I, Gottlieb J, Warnecke G, Dettmer S, Laenger F, Jonigk D. Pulmonary Fibroelastotic Remodeling Revisited. Cells. 2021 Jun 1;10(6):1362. doi: 10.3390/cells10061362.
Jonigk D, Stark H, Braubach P, Neubert L, Shin HO, Izykowski N, Welte T, Janciauskiene S, Warnecke G, Haverich A, Kuehnel M, Laenger F. Morphological and molecular motifs of fibrosing pulmonary injury patterns. J Pathol Clin Res. 2019 Oct;5(4):256-271. doi: 10.1002/cjp2.141.
Since the first infections with the SARS-CoV-2 virus occurred in fall 2019, there have been around 760 million infections and around 7 million reported deaths worldwide (as of 03/2023). In recent years, this has posed an enormous challenge for national and international healthcare systems. The emergence of this novel viral infection and its consequences also posed a major scientific challenge.
While initially the high mortality rate in the acute phase was the leading problem due to the lack of background immunity in the global population, there is now a high number of people with various long-term consequences following a COVID infection.
Selected publications
Kamp JC, Werlein C, Plucinski EK, Neubert L, Welte T, Lee PD, Tafforeau P, Walsh C, Kuehnel MP, Schuppan D, Hoeper MM, Jonigk DD, Ackermann M. Novel Insight into Pulmonary Fibrosis and Long Covid. Am J Respir Crit Care Med. 2023 Feb 2. doi: 10.1164/rccm.202212-2314LE.
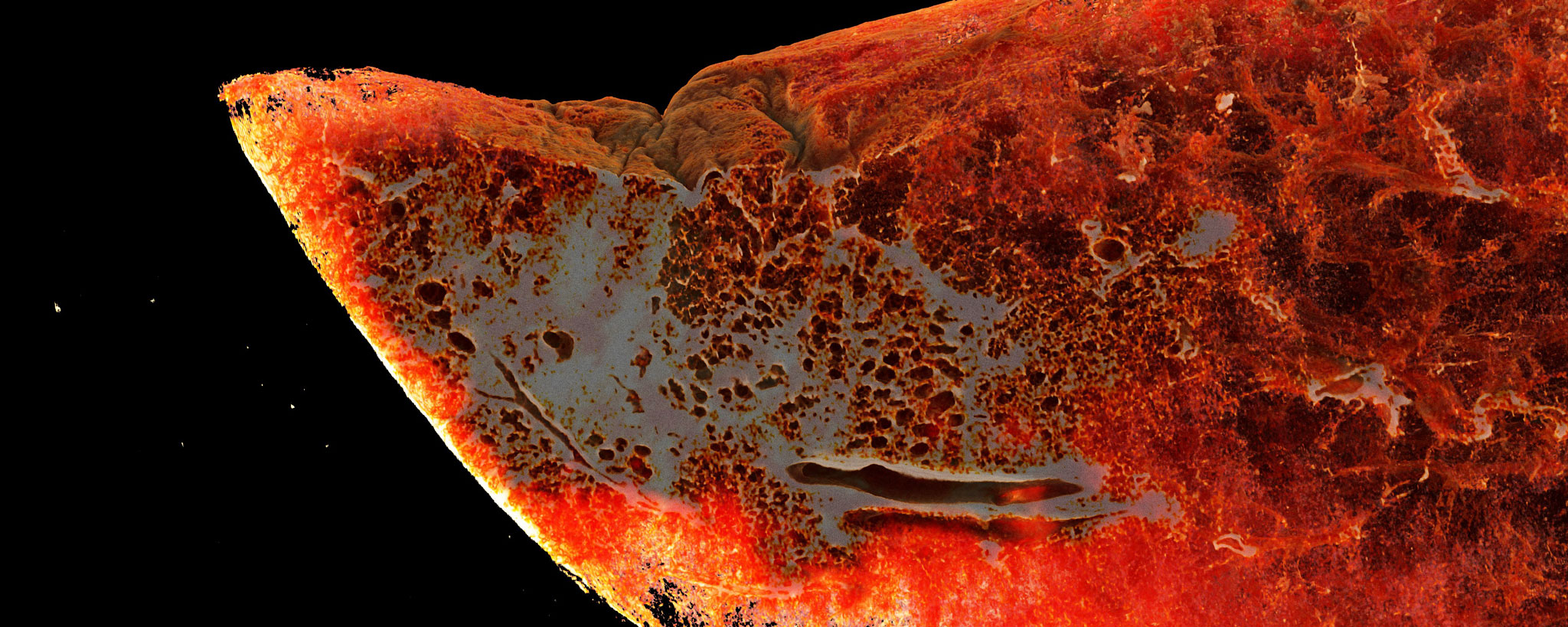
Ackermann M, Kamp JC, Werlein C, Walsh CL, Stark H, Prade V, Surabattula R, Wagner WL, Disney C, Bodey AJ, Illig T, Leeming DJ, Karsdal MA, Tzankov A, Boor P, Kühnel MP, Länger FP, Verleden SE, Kvasnicka HM, Kreipe HH, Haverich A, Black SM, Walch A, Tafforeau P, Lee PD, Hoeper MM, Welte T, Seeliger B, David S, Schuppan D, Mentzer SJ, Jonigk DD. The fatal trajectory of pulmonary COVID-19 is driven by lobular ischemia and fibrotic remodelling. EBioMedicine. 2022 Nov;85:104296. doi: 10.1016/j.ebiom.2022.104296.
Werlein C, Ackermann M, Stark H, Shah HR, Tzankov A, Haslbauer JD, von Stillfried S, Bülow RD, El-Armouche A, Kuenzel S, Robertus JL, Reichardt M, Haverich A, Höfer A, Neubert L, Plucinski E, Braubach P, Verleden S, Salditt T, Marx N, Welte T, Bauersachs J, Kreipe HH, Mentzer SJ, Boor P, Black SM, Länger F, Kuehnel M, Jonigk D. Inflammation and vascular remodeling in COVID-19 hearts. Angiogenesis. 2022 Nov 12:1-16. doi: 10.1007/s10456-022-09860-7.
Kamp JC, Neubert L, Ackermann M, Stark H, Werlein C, Fuge J, Haverich A, Tzankov A, Steinestel K, Friemann J, Boor P, Junker K, Hoeper MM, Welte T, Laenger F, Kuehnel MP, Jonigk DD. Time-Dependent Molecular Motifs of Pulmonary Fibrogenesis in COVID-19. Int J Mol Sci. 2022 Jan 29;23(3):1583. doi: 10.3390/ijms23031583.
Ackermann M, Verleden SE, Kuehnel M, Haverich A, Welte T, Laenger F, Vanstapel A, Werlein C, Stark H, Tzankov A, Li WW, Li VW, Mentzer SJ, Jonigk D. Pulmonary Vascular Endothelialitis, Thrombosis, and Angiogenesis in Covid-19. N Engl J Med. 2020 Jul 9;383(2):120-128. doi: 10.1056/NEJMoa2015432.
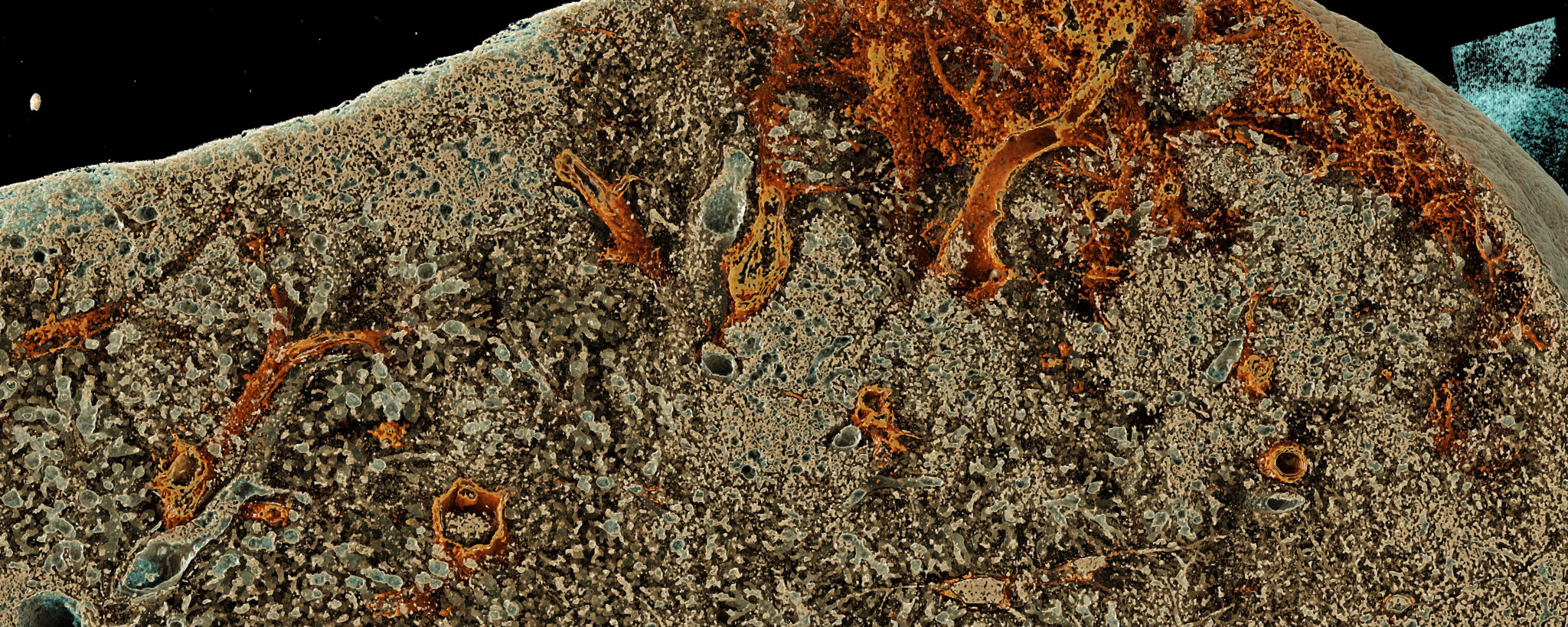
In the end-stage of various lung diseases, such as cystic fibrosis (CF), pulmonary fibrosis, chronic obstructive pulmonary disease (COPD) or pulmonary arterial hypertension (PAH), a lung transplant is sometimes the only remaining treatment option.
However, there is a substantial risk of various complications following lung transplantation. In addition to direct toxic effects due to the necessary drug immunosuppression, acute rejection reactions, serious infections or chronic graft dysfunction (CLAD) can occur, for example. The prediction of the long-term course and possible complications is currently the subject of research.
Selected publications
Jonigk D, Rath B, Borchert P, Braubach P, Maegel L, Izykowski N, Warnecke G, Sommer W, Kreipe H, Blach R, Anklamm A, Haverich A, Eder M, Stadler M, Welte T, Gottlieb J, Kuehnel M, Laenger F. Comparative analysis of morphological and molecular motifs in bronchiolitis obliterans and alveolar fibroelastosis after lung and stem cell transplantation. J Pathol Clin Res. 2016 Dec 10;3(1):17-28. doi: 10.1002/cjp2.60.
Jonigk D, Izykowski N, Rische J, Braubach P, Kühnel M, Warnecke G, Lippmann T, Kreipe H, Haverich A, Welte T, Gottlieb J, Laenger F. Molecular Profiling in Lung Biopsies of Human Pulmonary Allografts to Predict Chronic Lung Allograft Dysfunction. Am J Pathol. 2015 Dec;185(12):3178-88. doi: 10.1016/j.ajpath.2015.08.016.
AG Lungenforschung maintains a close research partnership with Prof. Dr. Danny D. Jonigk and PD Dr. Mark Kühnel from the Institute of Pathology at RWTH Aachen University. Prof. Jonigk was a founding member of the Lung Research Group at the MHH and its scientific director for many years. In September 2022, he accepted the call to RWTH Aachen University, where he has been Director of the Institute of Pathology ever since. Dr. Kühnel can also look back on many years of commitment as a laboratory manager in the Lung Research Group. In March 2023, he also moved to RWTH Aachen University, where he is now working with Mr. Jonigk to further develop the existing research infrastructure. Both scientists will remain members of the DZL site BREATH. We look forward to an intensive collaboration!
Head of working group


Group members





















Research partnership RWTH Aachen University



Further information
- Johanna Otto
- Chrissi Meyer
- Berenice Rath
- Lavinia Neubert
- Torsten Lippmann
- Judith Wehling
- Elias Mund
- Paul Borchert
- Eike Preuß