Projects under the direction of Dr. Alexandra Koch
Our research group investigates cellular Communications in the context of cancer. On the one hand, we are interested in direct cell-cell contacts between cancer cells and the surrounding tissue, which we try to visualize with the help of three-dimensional culture systems. On the other hand, we work on signals of receptor tyrosine kinases and their function in healthy tissue and in cancer.
Our group studies cellular communication in the context of cancer. In one project, we are interested in direct cell-cell contacts between cancer cells and the tumor microenvironment which we are examining with the help of three-dimensional cell cultures. In a second project, we are investigating signals from receptor tyrosine kinases and their function in healthy tissue and in cancer.
Bachelor's and Master's theses: Feel free to contact us!
I offer a Master's thesis on the topic of 3D culture systems. You can find the announcement in ILIAS.
1) Cell-cell contacts in organoids
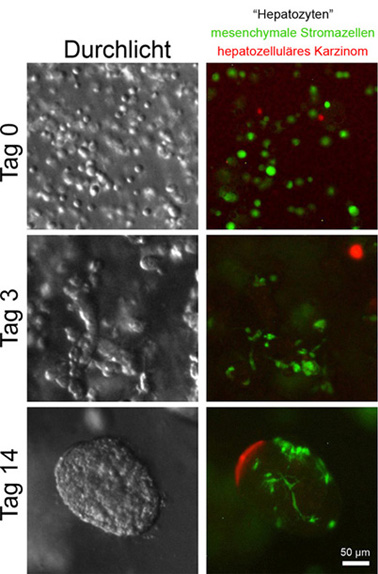
When cancer develops, the surrounding tissue (tumor microenvironment) first tries to suppress tumor growth. If the tumor overcomes these mechanisms, it changes the tissue environment in such a way that it supports its growth. Our research group investigates the Communications between cancer cells and the surrounding tissue using three-dimensional culture systems composed of different cell types (3D co-culture, organoid). These serve as tools to identify and better understand direct contacts between cells. A mixture of non-transformed cell types and a cancer cell line is poured into extracellular matrix. The cells form contacts with neighboring cells and organize themselves in relation to each other. It was found that individual cell lines behaved very differently in the 3D co-culture than in the Petri dish. They suddenly required media additives or extracellular matrix components for their survival, which were not necessary in the Petri dish. Depending on the medium, the morphology of the cells changed or the same cell lines either moved away from each other or formed a cluster. The three-dimensional culture shape (including interaction with the extracellular matrix and mechanical properties) apparently has profound effects on cell behavior, which cannot be represented in the Petri dish, but of course plays a role in the body.
During cancer development, the surrounding tissue (tumor microenvironment) initially tries to suppress tumor growth. If the tumor can overcome these mechanisms, it changes the microenvironment in such a way that it supports tumor growth. Our group investigates the communication between cancer cells and the surrounding tissue using a three-dimensional culture system consisting of multiple cell types (3D co-culture, organoid). We utilize this system as a tool to identify and characterize direct contacts between cells. A mixture of non-transformed cell types and a cancer cell line is embedded into extracellular matrix. The cells form contacts with neighboring cells and organize themselves into three-dimensional structures. Interestingly, the behavior of individual cell lines differed strongly between 3D co-culture and regular culture in the petri dish. The cells suddenly required media supplements or extracellular matrix components for their survival that were not necessary in the petri dish. Depending on the medium, cell morphology changed or the same cell lines either actively separated from one another or assembled into 3D structures. The three-dimensional culture (including interaction with the extracellular matrix and mechanical properties) apparently has profound effects on cell behavior which cannot be reproduced in the petri dish.
2) Signals of the intracellular domain of CSF-1R
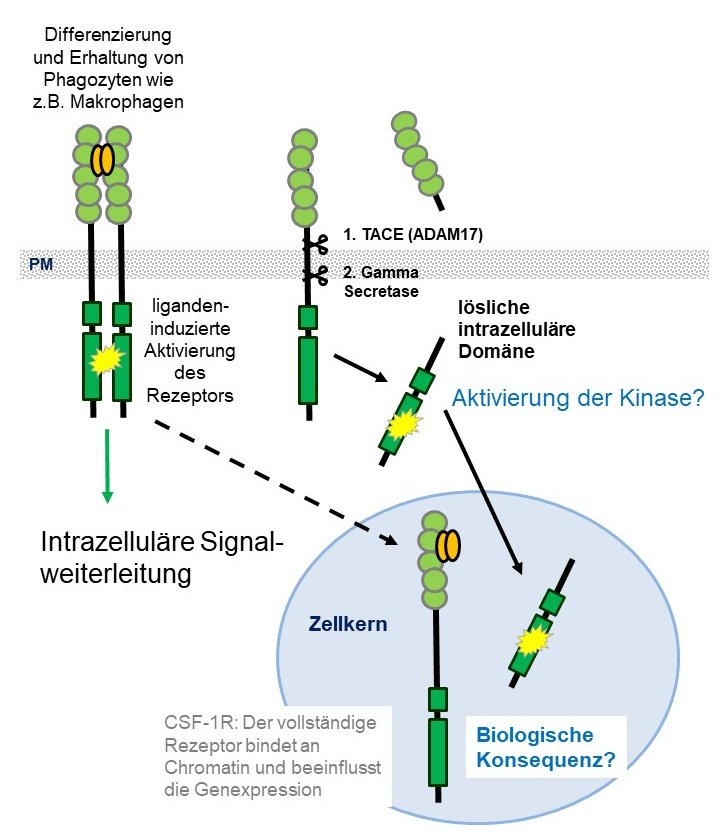
Receptor tyrosine kinases are often found as "oncogenic drivers" in mutated form or aberrantly expressed in many types of cancer. Their signal, triggered by the appropriate ligand from the plasma membrane, is already well characterized. In some cases, however, proteolytic cleavage releases the intracellular kinase domain in the cell. In addition, both complete receptors and the intracellular domains are found in the cell nucleus. Whether and how signaling via these pathways differs from classical ligand-induced signaling is poorly understood, as is their role in cancer cells. An example of such signaling is the macrophage colony-stimulating factor receptor CSF-1R, which is normally only found in phagocytes such as macrophages, but is also expressed in epithelial cancer cells. We have already shown that the receptor in epithelial cancer cells could contribute to their survival under certain anticancer drugs. We are currently investigating the mechanism of how the intracellular domain enters the cell nucleus and whether its kinase function plays a role there.
Mutated or aberrantly expressed receptor tyrosine kinases can act as oncogenic drivers in many types of cancer. Their signal, triggered by the appropriate ligand from the plasma membrane, is already well characterized. However, proteolytic cleavage events may release the intracellular kinase domain of several receptor tyrosine kinases. In addition, both complete receptors and the intracellular domains are found in the cell nucleus. Whether and how signals via these pathways differ from classical ligand-induced signaling is poorly understood, as is their role in cancer cells. An example of such signals is the macrophage colony stimulating-factor receptor, CSF-1R, which is normally expressed mainly in phagocytes such as macrophages, but is also aberrantly expressed in epithelial cancer cells. We have already shown that the receptor in epithelial cancer cells could contribute to their survival under certain anticancer drugs. At the moment we are investigating the mechanism by which the intracellular domain can enter the nucleus and a potential biological role of its nuclear kinase activity.