Current research interests
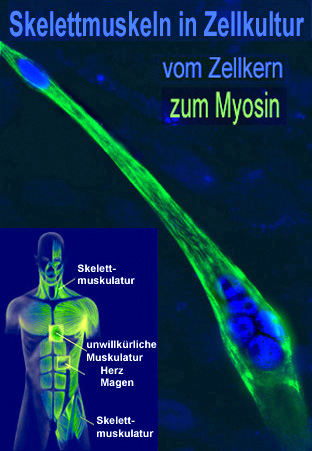
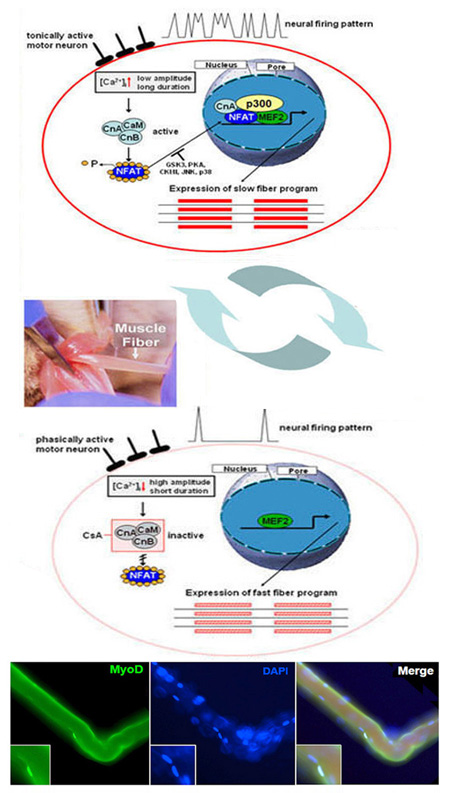
Multinucleated adult skeletal muscle cells develop in a complex differentiation process from mononuclear precursor cells, the myoblasts. The adult skeletal muscle cells (myotubes) consist of various muscle fibers. They are characterized by a pronounced plasticity. This plasticity, also known as transformation or fiber type shift, is one of the outstanding features of skeletal muscle. It occurs during changes in physiological requirements, such as endurance training, but also during inactivity-induced immobility, ageing, obesity, muscle diseases (Duchenne muscular dystrophy) or non-muscular diseases (type II diabetes). A fast muscle fiber (high ATPase activity; fast fatigability) can be converted into a slow muscle fiber (low ATPase activity; low fatigability) under continuous stress. The changes in the intracellular calcium concentration ([Ca2+]i) are of great importance here. Its increase activates the calcium-dependent phosphatase calcineurin and calcineurin-dependent signal transduction pathways in the muscle. Dephosphorylation and activation of the transcription factor NFAT (isoform c1), as well as the subsequent nuclear translocation, play an important role in the formation of the slow fiber-type-specific gene program. In addition to dephosphorylation, we could show that NFATc1 is altered by further posttranslational modifications (PMTs).
Furthermore, mitogen-activated protein kinases (MAPKs), in particular p38 MAPK and possibly also MAPK-activated protein kinases 2 and 3 (MK2/3), are involved as regulators in muscle differentiation processes and in the maintenance of fast muscle fiber type. The extent to which these two signal transduction pathways interact is being investigated. As far as is known, a decrease in [Ca2+]i , as well as the activation of p38 MAPK (isoform a/b) and the transcription factor MEF2C, causes the gene expression of the fast fiber type-specific heavy chain of myosin IId/x.
Examples of medical relevance:
Fiber type transformation currently plays a role in the therapy of cardiac insufficiency (cardiac assist), in the therapy of inactivity-induced muscle atrophy, e.g. in bedridden patients, and in the treatment of muscle wasting in old age, which leads to muscle weakness and a tendency to fall.
Scientific questions:
1) Studies on the physiological function of MAPK-activated protein kinases 2 and 2 (MK2/3) in the skellet muscle and heart of double-knockout mice and in the muscle cell line C2C12;
2) Influence of posttranslational modifications (acetylation/ SUMOylation) of the different on the regulation of target genes in skeletal/cardiac muscle .
Laboratory methods: cloning, promoter analysis, pull-down assay, EMSA, RNA silencing, mutagenesis, ChIP, muscle cell culture, etc.