AG Epigenomics
Head: Dr. rer. nat. Britta Skawran
"Hepatocellular carcinoma (HCC) is a highly malignant tumor with limited therapeutic options. Understanding the molecular mechanisms of hepatocarcinogenesis is of great importance for the selection of new therapeutic targets. It has recently become known that the development of a tumor and its progression are not only caused by genetic changes within the cell, but that epigenetic features such as histone acetylation are also disturbed.", Britta Skawran
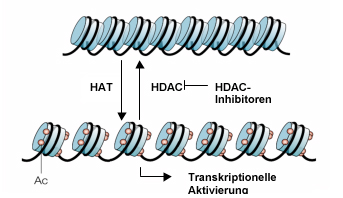
Model for the regulation of chromatin remodeling and transcriptional activity mediated by reversible histone acetylation; modified from (P. A. Marks et al., 2001)
Acetylation (Ac) of the lysine residues of the amino-terminal ends of histone proteins loosens the contact between histone proteins and DNA and thus leads to transcriptional activation in the areas concerned. Inhibition of the histone deacetylases HDACs by HDAC inhibitors leads to hyperacetylation.
Staff members
-
Dr. rer. nat. Beate Vajen, Post-Doc
-
Marlies Eilers, MTA
-
Vera Schäffer, biology laboratory assistant
Our research objective
Our aim is to elucidate the functional consequences of altered mRNA and miRNA expression for the development and progression of HCC by histone deacetylation and acetylation.
In previous studies, we were able to show that several histone deacetylases are significantly overexpressed in HCC. It is known that alteration of histone acetylation in human tumor cells leads to altered transcription of genes and miRNAs. Using cell culture work and modern molecular biology methods such as array CGH, expression analysis and proteomics, we can identify deregulated signaling pathways in the cell and possibly find targets for new therapeutic options.
Our main areas of research
In human tumor cells, the chromatin structure of further genomic regions is altered by epigenetic modification, such as histone methylation or histone acetylation. The loss of the acetyl residue by histone deacetylases (HDAC) leads to a strong condensation of the chromatin, which is accompanied by an inhibition (blockade) of transcription. Thus, the alteration of histone acetylation leads to altered transcription of genes and miRNAs that regulate proliferation (growth), apoptosis (programmed cell death), differentiation and genetic stability. As early as 2005, Fraga et. al. were able to attribute a fundamental function in the development of cancer to the loss of acetylation at lysine 16 of histone H4.
It has also recently become known that the expression of microRNAs is strongly influenced by histone modifications.
The subject of our research is the epigenetic changes in HCC. We are particularly interested in the context in which they occur and what significance they have for malignant cell transformation. We are investigating the effect of altering histone acetylation in the cell culture system. The focus is on the tumor-suppressive and oncogenic properties of epigenetically influenced genes and miRNAs.