AG Scholz
MAPs Single Molecule Motility Group / Single Molecule Motility of Motor Proteins
Research focus
One focus of the group is the functional characterization of kinesins, myosins and dynein at the single molecule level. The intracellular transport e.g. in nerve cells, the separation of replicated chromosomes during cell division, the beating of flagella and cilia as well as the muscle contraction of multicellular organisms are examples of movements of and in living organisms. They are based on the cyclic interaction of the motor proteins kinesin, myosin and dynein with structural proteins of the cytoskeleton such as actin and tubulin.
Motor proteins of the kinesin family are microtubule-activated ATPases. They move along microtubules, tubular structures of the cytoskeleton. These are formed from protofilaments created by polymerization of α/β-tubulin heterodimers. Conventional kinesin (also kinesin-1, Figure 1) was originally discovered in giant axons of squid, where it is responsible for vesicle transport into the cell periphery of neurons. In contrast to muscular myosin-2, conventional kinesin is a processive motor molecule. This means that it can take hundreds of 8 nm long steps, corresponding to the size of an α/β-tubulin heterodimer, along a microtubule without dissociating from it. Presumably, each step is coupled to the hydrolysis of a molecule of ATP. The ability of kinesin to travel long distances along microtubules without dissociating from them is extremely important for its biological function as a long-distance transporter. However, it has not yet been clarified in detail how kinesin molecules coordinate their two motor domains for this purpose or how the direction of travel of kinesin molecules is determined.
Measurements at the level of individual molecules offer possibilities for investigating these questions. TIRF (Total Internal Reflection Fluorescence) or evanescent wave microscopy is a very suitable technique for this. It can be used, for example, to detect individual fluorescently labeled ATP and motor molecules and track their movements. In this way, the movement of individual motor molecules can be correlated with the binding and hydrolysis of fluorescently labeled ATP. These assays also allow us to characterize the functional effects of specifically introduced mutations in the motor proteins. This allows us to deduce the functional relevance of individual structural elements as well as the structural basis of Communications between the individual domains of the motor proteins.

Movie 1: Single molecule assay kinesin-1 on a microtubule
Movie 1 shows an example of the ATP-driven processive movements of single, fluorescently labeled kinesin molecules (green) on an immobilized, also fluorescently labeled microtubule (red).
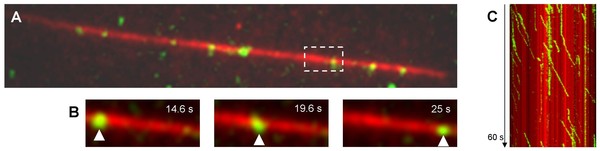
Fig. 2: (A) Snapshot of single moving kinesin-1 molecules (green) along an immobilized labeled microtubule (length 34 µm, red) in the presence of ATP. (B) A single kinesin molecule (green) moves processively along the immobilized microtubule. (C) Legal movements of the individual kinesin molecules can be recognized in a position-time plot (kymograph) as lines deviating to the right (green) (running speed 500-600 nm/s).
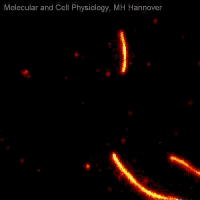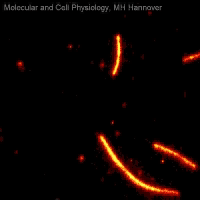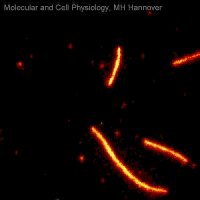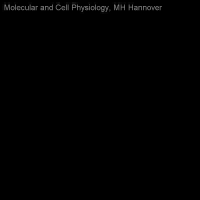
Movie 2: Microtubule sliding assay
Movie 2 shows a microtubule sliding assay in which fluorescently labelled microtubules (red) are moved on a lawn of immobilized kinesin molecules (not visible) while consuming ATP.
- Dr. rer. nat. Tim Scholz
- Petra Uta (Medical Technologist for Laboratory Analysis)
- Marina Zefi
Scientific work
final theses
- Zefi, Marina
BSc Biologie, Einfluss von Fluoreszenzmarkierungen und Temperaturen auf die in vitro Gleitgeschwindigkeit von Aktinfilamenten auf kardialen Myosinisoformen des Schweins - Okon, Adrianne
BSc Biologie, Funktionelle Charakterisierung kardialen Myosins mittels eines in vitro Motilitätsassays - Mohebbi, Maral
Dr. med. dent., Einfluss der essentiellen leichten Myosinkette auf die Aktinfilament-Gleitgeschwindigkeit von β-Myosin“. - Heilmann, Pia Louisa
BSc Biologie, Funktioneller Vergleich der Temperaturabhängigkeit kardialer Myosinisoformen unter Verwendung eines in vitro Motilitätsassays - Osten, Jennifer
Dr. rer. nat., Functional characterization of different myosin isoforms and disease-associated mutations in the cardiac β-myosin motor domain - Huhnt, Helen Elisabeth
Dr. med. dent., Auswirkungen der Mutation S456Y in der Schalter-II-Region von Myosin II auf den ATP-Umsatz einzelner Moleküle - Jalal-Ebrahimi, Avesta
Dr. med. dent., Einfluss des Nukleotidstatus von Tubulin auf die Tau-Mikrotubuli-Interaktion - Werkman, Christoph
Dr. med., Funktionelle Auswirkungen der Punktmutation R723G in der Myosin-Kopfdomäne bei familiärer hypertropher Kardiomyopathie Untersuchung am Einzelmolekül mittels optischer Falle - Lesch, Elisa
Dr. med. dent., Auswirkungen der krankheits-assoziierten Tau-Protein-Mutation ΔK280 auf die Kinesinfunktion - Pfeffer, Tobias J.
Dr. med., Inhibition von Krankheits-assoziierten Kinesinmolekülen - Hinrichs, Maike
Dr. rer. nat., Diffusion von Tau auf Mikrotubuli und Auswirkung auf die Kinesin-Funktion - Hanke, Eva
M.Sc., Molekulare Medizin, Establishment of a cardiac in vitro motility test system for the functional characterisation of left ventricular myosin molecules - Jablonski, Janos
B.Sc.Biologie, Direkte Beobachtung des ATP-Umsatzes am individuellen Myosinmolekül mittels TIRF-Mikroskopie - Pliquet, Julia
B.Sc. Biologie, Kinesin 5 ‐ Funktion und Inhibition, eine Einzelmolekülstudie - Koch, Peter
Dipl. Biochemiker, Untersuchung eines Kinesinkonstrukts auf Einzelmolekülebene mittels TIRF-Mikroskopie - Lampe, Marko
Dipl. Biochemiker, Charakterisierung der Fortbewegung von Mikrotubulus-gebundenen Kinesinen auf Einzelmolekülebene mittels Evanescent Field Mikroskopie
You can find out more about teaching in physiology for human dentistry, biology, biomedicine and biochemistry here.
- Rajendraprasad G, Kyriazi D, Franz P, Bader A, Erent M, Uta P, Preller M, Scholz T, Tsiavaliaris G, Long-tailed class I myosins rely on tail-mediated phosphoinositide recognition for specific membrane recruitment, .,Cell Commun Signal. 2025 Dec 4;23(1):519. doi: 10.1186/s12964-025-02528-x. PubMed
- Osten, J., Mohebbi, M., Uta, P., Matinmehr, F., Wang, T., Kraft, T., Amrute-Nayak, M., Scholz, T. (2022) Myosin essential light chain 1sa decelerates actin and thin filament gliding on β-myosin molecules. J Gen Physiol. Oct 3; 154(10):e202213149. Doi: 10.1085/jgp.202213149.
- Wang, T., Spahiu, E., Osten, J., Behrens, F., Gruenhagen, F., Scholz, T., Kraft, T., Nayak, A., Amrute-Nayak, M. (2022) Cardiac ventricular myosin and slow skeletal myosin exhibit dissimilar chemo-mechanical properties despite bearing the same myosin heavy chain isoform. J Biol Chem. 2022 May 24:102070. doi: 10.1016/j.jbc.2022.102070.
- Amrute-Nayak, M., Nayak, A., Steffen, W., Tsiavaliaris, G., Scholz, T., Brenner, B. (2019) Transformation of the non-processive fast skeletal myosin II into a processive motor. Small. Jan 18:e1804313. Doi: 10.1002/smll.201804313.
- Behrens, V.A., Walter, W.J., Peters, C., Wang, T, Brenner, B., Geeves, M.A., Scholz, T.*, Steffen, W.* (2018) Mg2+-free ATP regulates native cytoplasmic dynein's processivity. FEBS Letters. Doi: 10.1002/1873-3468.13319. (*shared last authorship)
- Pfeffer, T.J., Sasse, F., Schmidt, C.F., Lakämper, S., Kirschning, A., Scholz, T. (2016) The natural diterpene tonantzitlolone A and its synthetic enantiomer inhibit cell proliferation and kinesin-5 function. Eur J Med Chem. 112:164-170. doi: 10.1016/j.ejmech.2016.02.022.
- Scholz, T. # and Mandelkow, E. (2014) Transport and diffusion of Tau protein in neurons. Cell Mol Life Sci. 71, 3139-50 [Epub 2014 Apr 1 as doi: 10.1007/s00018-014-1610-7] (#corresponding author)
- Amrute-Nayak, M., Lambeck,K.-A., Radocaj, A., Huhnt, H.E., Scholz, T., Hahn, N., Tsiavaliaris, G., Walter, W.J., Brenner, B. (2014) ATP turnover by individual myosin molecules hints at two conformers of the myosin active site. Proc Natl Acad Sci USA. 111(7):2536-41. doi: 10.1073/pnas.1316390111.
- Hinrichs, M.H., Jalal, A., Brenner, B., Mandelkow, E., Kumar, S., Scholz, T. (2012) Tau protein diffuses along the microtubule lattice. J Biol Chem. 287, 38559-68.
- Brenner, B., Hahn, N., Hanke, E., Martinmehr, F., Scholz, T., Steffen, W., Kraft, T. (2012) Mechanical and kinetic properties of β-cardiac/slow skeletal muscle myosin. J Muscle Res Cell Motility. Doi:10.1007/s10974-012-9315-8.
- Rump, A.*, Scholz, T.*, Thiel, C., Hartmann, F.K., Uta, P., Hinrichs, M.H., Taft, M.H., Tsiavaliaris, G. (2011) Myosin-1C associates with microtubules and stabilizes the mitotic spindle during cell division. J Cell Sci. 124, 2521-8 (*shared first authorship)
- Comment in "In this issue: Myosin-1C gives spindles support" J Cell Sci. (2011) 124:e1501.
- Scholz, T., Vicary, J.A., Jeppesen, G.M., Ulcinas, A., Hörber, J.K.H., Antognozzi, M. (2011). Processive behavior of kinesin observed using micro-fabricated cantilevers. Nanotechnology. 22,095707. doi: 10.1088/0957-4484/22/9/095707
- Comment in Demming, A. "Editorial: Nanodevices come to life" Nanotechnology 22 (2011) 090201 (2pp).
- Radtke, K., Kieneke, D., Wolfstein, A., Michael, K., Steffen, W., Scholz, T., Karger, A., Sodeik, B. (2010). Plus- and Minus-end Directed Microtubule Motors Bind Simultaneously to Herpes Simplex Virus Capsids using Different Inner Tegument Structures. PLoS Pathog 6, e1000991. Doi:10.1371/journal.ppat.1000991.
- Amrute-Nayak, M., Antognozzi, M., Scholz, T., Kojima, H., Brenner, B. (2008). Inorganic phosphate binds to the empty nucleotide binding pocket of conventional myosin II. J Biol Chem. 283, 3773-3781.
- Scholz, T., Altmann, S.M., Antognozzi, M., Tischer, C., Hörber, J.K.H., Brenner, B. (2005). Mechanical properties of single myosin molecules probed with the Photonic Force Microscope. Biophys J. 88, 360-371.
- Becker, N.B., Altmann, S.M., Scholz, T., Hörber, J.K.H., Stelzer, E.H.K., Rohrbach, A. (2005). Three-dimensional bead position histograms reveal single-molecule nanomechanics. Physical Review E. 71, 021907 (7 pages).
- Scholz, T. and Brenner B. (2003). Actin sliding on reconstituted myosin filaments containing only one myosin heavy chain isoform. J Muscle Res Cell Motility. 24, 77-86.
- Köhler, J., Winkler, G., Schulte, I., Scholz, T., McKenna, W., Brenner, B., Kraft, T. (2002). Mutation of the myosin converter domain alters cross-bridge elasticity. Proc Natl Acad Sci USA. 99, 3557-3562.
- Influences of the tau protein associated with Alzheimer's disease on kinesin function, Collaborators: Müller HS, Jalal A, Uta P; Cooperation: Mandelkow, E, MPI-ASMB, Hamburg; Manstein D, Biophysical Chemistry, MHH
- Mechanisms of action of small molecule inhibitors on mitotic kinesin molecules, Collaborators: Pfeffer T; Cooperation: Kirschning A, Institute of Organic Chemistry, LUH; Schmidt CF, Georg-August-Universität Göttingen
- Class 1 myosins as regulators of microtubule dynamics and kinesin function, Collaborator: Uta P; Cooperation: Tsiavaliaris G, Biophysical Chemistry, MHH
- Mechanical characterization of single kinesin molecules with the Lateral Molecular Force Microscope and the Photonic Force Microscope, Cooperation: Antognozzi M, Hörber H, University of Bristol, UK