Project presentation 2020
cAMP-dependent regulation of HCN4 controls the tonic entrainment process in sinoatrial node pacemaker cells
Fenske S, Hennis K, Roetzer R, Brox V, Becirovic E, Scharr A, Gruner C, Ziegler T, Mehlfeld V, Brennan J, Efimov I, Pauza A, Moser M, Wotjak C, Kupatt C, Goenner R, Zhang R, Zhang H, Zong X, Biel M*, Wahl- Schott* C.
Pacemaker channels are representatives of hyperpolarization-activated and cyclic nucleotide-gated (HCN) cation channels and are considered essential motors for the generation of the heartbeat in the sinoatrial node (SAN) of the heart. Four representatives, HCN1-HCN4, are found in humans and mice. HCN4 is the main isoform and is expressed throughout the SAN. HCN channels are opened by hyperpolarization. In addition, activation of the channels is regulated by cyclic adenosine monophosphate (cAMP). An increase in the intracellular cAMP concentration, as occurs when the sympathetic nervous system is activated, leads to an increase in HCN channel activity. In the Research Report 2021, we present a project that is being carried out in the group of physiologist and pharmacologist Christian Wahl-Schott from the Institute of Neurophysiology at the MHH in collaboration with the pharmacologists Stefanie Fenske and Martin Biel of the Chair Pharmacology for Natural Sciences at the Department of Pharmacy at LMU Munich and published in the journal Nature Communications. In the study, the authors discovered how the secondary messenger cAMP regulates the heartbeat via an effect on HCN4 channels.
To investigate the role ofcAMP-dependent regulation(CDR) of HCN4, the research team produced knock-in mice in which cAMP can no longer bind to HCN4 (HCN4FEA mouse line). When studying individual pacemaker cells from the SAN, the scientists discovered that SAN cells can not only adopt a long-known activity mode in which the cells fire action potentials and drive the heartbeat(firing mode), but can also adopt a non-firing mode in which the cells stand still for a period of up to one minute. In the network of the sinus node, the number of cells in non-firing mode is set by the CDR. Pacemaker cells in non-firing mode act as "brakes" in the SAN network, inhibiting the activity of neighboring pacemaker cells in firing mode. It is known that inhibitory elements generally increase the stability of electrically active networks. In the brain, for example, inhibitory neurons stabilize the activity of neuronal network connections.
Video: Beating atrium with sinus node region in a preparation from the mouse.
The current work shows that inhibitory control of excitability is also required in the sinus node for stable pacemaker function. Both the overactivity and the lack of inhibitory control lead to a massive disturbance of the pacemaker function of the heart, which manifests itself as "sick sinus node" syndrome (see figure). The CDR of HCN4 can be used to set the exact dose for inhibition within the sinus node according to the situation. As a result, the heart rate can be effectively stabilized and both bradycardia and tachyarrhythmia can be counteracted.
In summary, this study shows that the CDR of HCN4 is important for the control of the SAN by the autonomic nervous system, in particular for a safe transition from a stable baseline heart rate to a new target rate during sympathetic and/or parasympathetic activity. The CDR of HCN4 appears to be particularly important in fine-tuning the heart rate-lowering effect of the parasympathetic nervous system. The CDR counteracts parasympathetic overdrive, an inappropriate drop in heart rate and the occurrence of bradycardia.
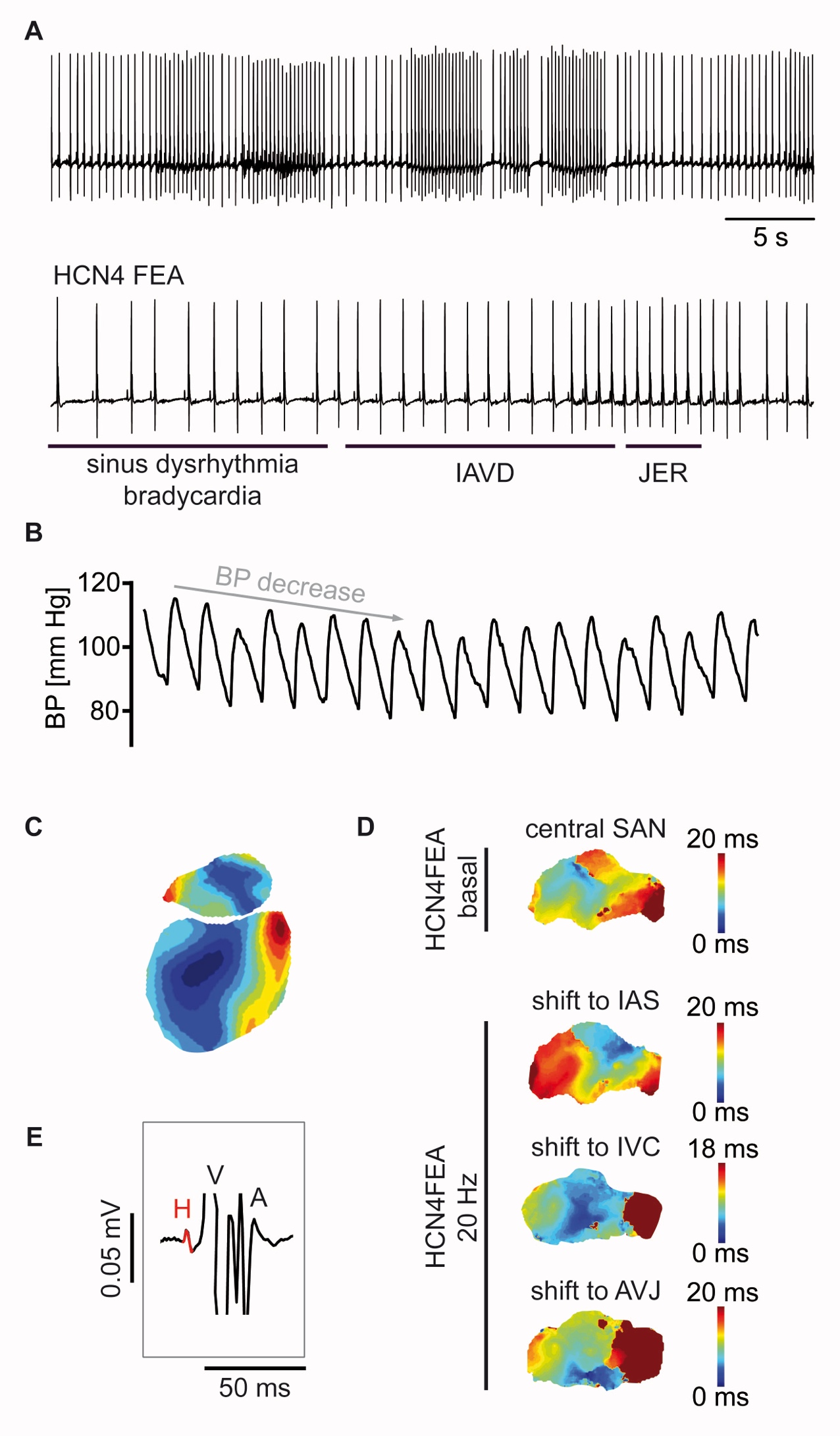
Figure: cAMP stabilizes the heartbeat via a direct effect on HCN channels and increases the precision of dynamic heart rate regulation by the autonomic nervous system. A, Absence of cAMP-dependent regulation (CDR) of HCN4 pacemaker channels in HCN4FEA knock-in mice leads to bradycardia, sinus dysrhythmia, and secondary arrhythmias such as isorhythmic AV dissociation (IAVD) and junctional escape rhythms (JER) as well as junctional tachycardia. These arrhythmias lead to a hemodynamically relevant reduction in cardiac output and to drastic fluctuations in arterial blood pressure(B). C, Optical mapping of excitation propagation in the whole heart of a wild-type (WT) mouse. D, Optical mapping of excitation propagation in the sinus node. Top: regular propagation of electrical excitation in a sinus node preparation of a HCN4FEA mouse. The blue region represents the location of the earliest excitation in the sinus node (leading pacemaker region, LPR). In WT mice, the localization of the LPR is stable (not shown). Bottom: In sinus node preparations from HCN4FEA mice, there are dynamic shifts of the LPR, towards the intraatrial septum (IAS), the inferior vena cava (IVC) and/or the AV junction (AVJ). This indicates unstable excitation formation in the sinus node of HCN4FEA mice. E, intracardiac in vitro ECG with His bundle electrogram to differentiate and quantify phases of anterograde and retrograde AV conduction. In addition, the study used echocardiography, right heart catheterization to obtain intracardiac ECGs and to map the conduction system and programmed atrial and ventricular stimulation, left heart catheterization to quantify hemodynamic parameters, confocal calcium and voltage imaging, ECG and blood pressure telemetry, spectral analyses of heart rate variability and characterization of baroreflex sensitivity, combined with cellular techniques such as patch-clamp.
Link to the article
Nature Communications 2020 11:5555. doi: 10.1038/s41467-020-19304-9.
Publisher:
President of the MHH
Prof. Dr. med. Michael P. Manns
Dean of Research of the MHH
Prof. Dr. med. Frank M. Bengel
Editing and contact person:
Office of the Dean of Research at Hannover Medical School
Petra Linke
Phone: 0511/ 532- 6023
Fax: 0511/ 532- 6024
E-mail: linke.petra@mh-hannover.de
Design:
Digital Media, Hannover Medical School
Phone: 05 11/ 532- 2963
Online implementation:
Office of the Dean of Research, Hannover Medical School
Jan Tauwaldt
and
Petra Linke
Phone: 0511/ 532- 6023
Research report
Here you can find the research report created with the help of the Research Information System (FIS). As in previous years, we would like to take the opportunity here to explicitly present one project as a representative.
Research Information System (FIS)Here you can find further information on the Research Information System (FIS).
University bibliography