Project presentation 2021
In sero veritas: A new tool for research Hepatitis C virus vaccine research
HCV, a global threat
Around 58 million people worldwide are infected with the hepatitis C virus (HCV). If the infection is not treated, it can lead to serious liver diseases such as liver fibrosis, cirrhosis and liver cancer. Although effective treatments are available, many infected people remain undiagnosed or do not have access to appropriate treatment. As a result, around 300,000 people die each year as a result of chronic HCV infection and there are 1.5 million new infections each year. A vaccine that protects against infection is not available.
HCV variability complicates the development of effective vaccines
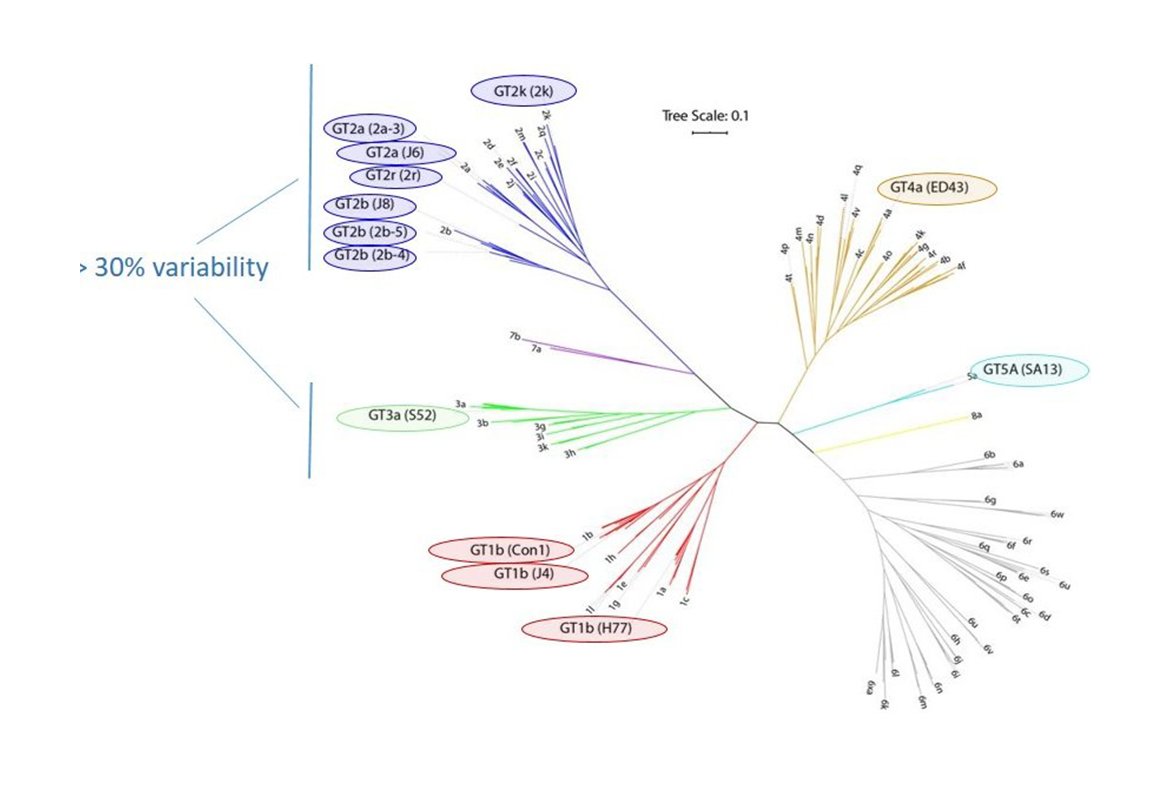
One of the reasons why it is so difficult to develop a vaccine against HCV is that the virus is extremely variable. In fact, different strains of the virus can differ at the sequence level at more than 30% of the positions.
The role of neutralizing antibodies in evaluating the efficacy of HCV vaccines
Many vaccines provide protection against viral infections because they elicit a strong antibody response. For this reason, analyzing the efficacy of antibodies is an important tool to assess the quality of a vaccine.
Hepatitis C reference viruses highlight potent antibody viral functional interactions
Video abstract
Objective: Neutralizing antibodies are key effectors of infection-induced and vaccine-induced immunity. Quantification of antibodies' breadth and potency is critical for understanding the mechanisms of protection and for prioritization of vaccines. Here, we used a unique collection of human specimens and HCV strains to develop HCV reference viruses for quantification of neutralizing antibodies, and to investigate viral functional diversity.
Conclusions: Representative isolates from six neutralization clusters broadly reconstruct the functional HCV neutralization space. They enable high resolution profiling of HCV neutralization and they may reflect viral functional and antigenic properties important to consider in HCV vaccine design.
HCV reference viruses for the evaluation of neutralizing antibodies
With a virus as variable as HCV, it is particularly important to test the effect of antibodies not only against a single HCV type. Otherwise, there is a risk of prioritizing very virus type-specific antibodies and overlooking particularly valuable antibody "all-rounders" that work well against many different virus types. But among the many HCV variants, what is the best way to select a few HCV types in order to measure broad antibody efficacy in a balanced way?
To answer this question, we first cloned 13 genetically very different HCV variants. These viruses belong to five of the eight most widespread HCV genotypes and therefore cover a very broad spectrum of the genetic diversity of the virus (Figure 1). In a next step, we used these viruses to perform neutralization tests with polyclonal antibodies from 101 HCV-positive patients infected with different HCV variants (Figure 2). These neutralization data were analyzed using a bioinformatic method (metric multidimensional scaling).
Illustrations
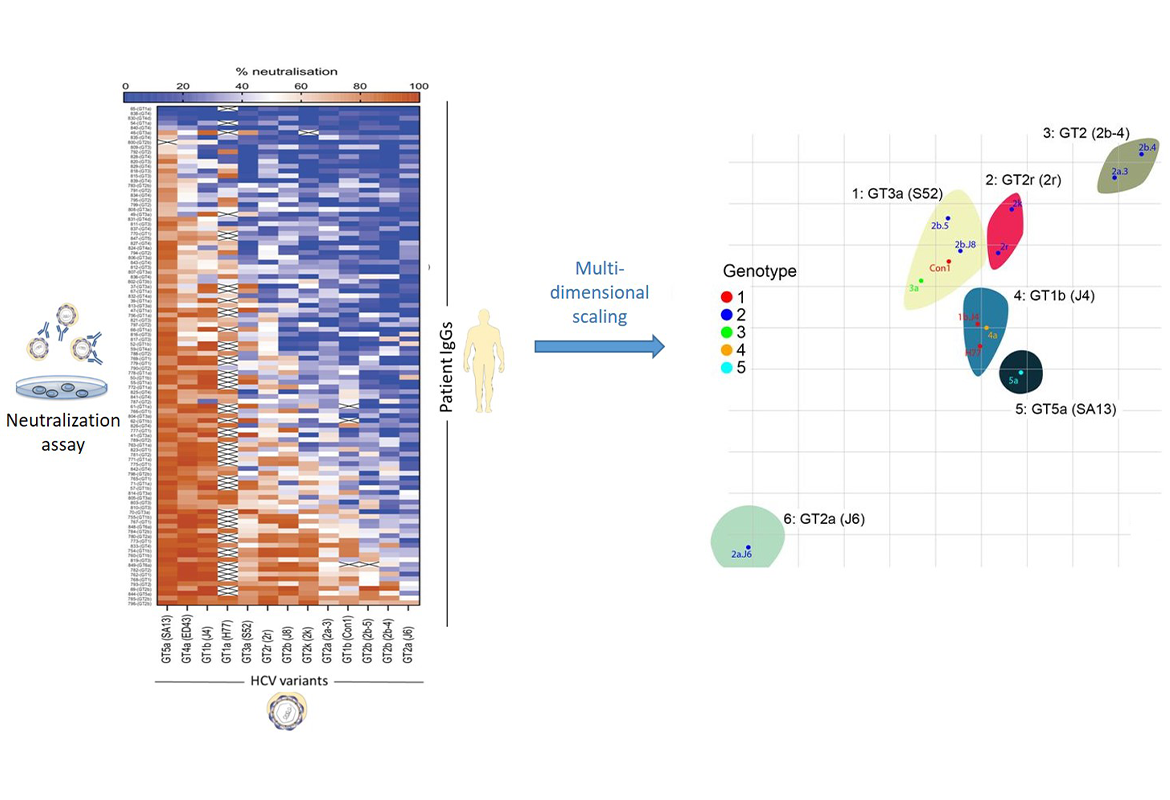
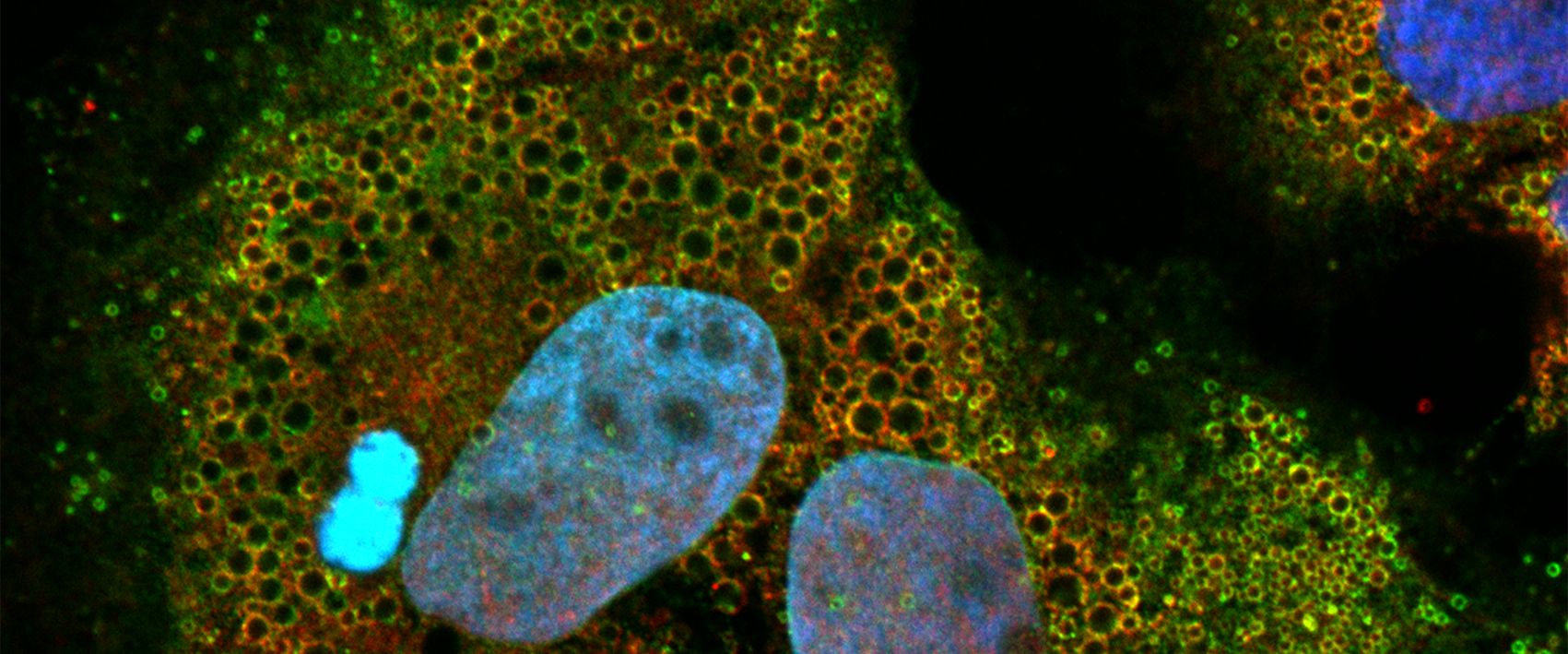
This analysis revealed that the virus strains studied can be divided into six different groups, which we have termed viral neutralization biotypes (NBTs). These NBTs cannot be predicted from genetic sequence comparisons, but represent different functional properties of the viral envelope proteins with respect to human antibodies (Bankwitz et al. Gut 2021).
In addition, we have used this method to identify patients who produce particularly good antibodies against the virus ("elite neutralizers") and to describe the properties of their antibodies (Weber et al. Immunity 2021).
Potential for the development of an HCV vaccine
These results advance the field of HCV vaccine research in two ways: On the one hand, we now have a robust test system and a gold standard for the detection of antibody responses.
On the other hand, this study has identified six neutralization groups (NBTs) that most likely represent different functional states of HCV E1/E2 proteins.
In order to develop broadly effective vaccines, it could be advantageous to consider these different envelope protein types of the six NBTs instead of simply using genetically different viruses as a vaccine cocktail.
Publications
Hepatitis C reference viruses highlight potent antibody responses and diverse viral functional interactions with neutralizing antibodies
Bankwitz D, Bahai A, Labuhn M, Doepke M, Ginkel C, Khera T, Todt D, Ströh LJ, Dold L, Klein F, Klawonn F, Krey T, Behrendt P, Cornberg M, McHardy AC, Pietschmann T. Gut. 2021 Sep;70(9):1734-1745. doi: 10.1136/gutjnl-2020-321190. Epub 2020 Dec 15. PMID: 33323394; PMCID: PMC8355883.
Analysis of antibodies from HCV elite neutralizers identifies genetic determinants of broad neutralizationWeber T, Potthoff J, Bizu S, Labuhn M, Dold L, Schoofs T, Horning M, Ercanoglu MS, Kreer C, Gieselmann L, Vanshylla K, Langhans B, Janicki H, Ströh LJ, Knops E, Nierhoff D, Spengler U, Kaiser R, Bjorkman PJ, Krey T, Bankwitz D, Pfeifer N, Pietschmann T, Flyak AI, Klein F. Immunity. 2022 Feb 8;55(2):341-35
Further information
Publisher:
President of the MHH
Prof. Dr. med. Michael P. Manns
Dean of Research of the MHH
Prof. Dr. med. Frank M. Bengel
Editing and contact person:
Office of the Dean of Research at Hannover Medical School
Petra Linke
Phone: 0511/ 532- 6023
Fax: 0511/ 532- 6024
E-mail: linke.petra@mh-hannover.de
Design:
Digital Media, Hannover Medical School
Phone: 05 11/ 532- 2963
Online implementation:
Office of the Dean of Research, Hannover Medical School
Jan Tauwaldt
and
Petra Linke
Phone: 0511/ 532- 6023
Research report 2021
Here you can find the research report created with the help of the Research Information System (FIS). As in previous years, we would like to take the opportunity here to explicitly present one project as a representative.
Research Information System (FIS)Here you can find further information on the Research Information System (FIS).
University bibliography- Project presentation 2021
- Project presentation 2020
- Project presentation 2019
- Project presentation 2018
- Project presentation 2017
- Research report 2016
- Research report 2015
- Research report 2014
- Research report 2013
- Research report 2012
- Research report 2011
- Research Report 2010
- Research Report 2009
- Research Report 2008
- Research Report 2007